Difference between revisions of "GlnA"
| Line 151: | Line 151: | ||
** GP247 (''glnA::cat''), available in [[Jörg Stülke]]'s lab | ** GP247 (''glnA::cat''), available in [[Jörg Stülke]]'s lab | ||
** BP148 (del(''[[glnR]]-[[glnA]]'')::''cat''), available in [[Fabian Commichau]]'s lab | ** BP148 (del(''[[glnR]]-[[glnA]]'')::''cat''), available in [[Fabian Commichau]]'s lab | ||
| + | ** GP1883 (del(''[[glnR]]-[[glnA]]'')::''ermC''), available in [[Fabian Commichau]]'s and [[Jörg Stülke]]'s labs | ||
* '''Expression vector:''' | * '''Expression vector:''' | ||
Revision as of 08:18, 24 September 2014
- Description: trigger enzyme: glutamine synthetase and effector of TnrA and GlnR
| Gene name | glnA |
| Synonyms | |
| Essential | no |
| Product | trigger enzyme: glutamine synthetase |
| Function | glutamine biosynthesis, control of TnrA and GlnR activity |
| Gene expression levels in SubtiExpress: glnA | |
| Interactions involving this protein in SubtInteract: GlnA | |
| Metabolic function and regulation of this protein in SubtiPathways: glnA | |
| MW, pI | 50 kDa, 4.874 |
| Gene length, protein length | 1332 bp, 444 aa |
| Immediate neighbours | glnR, ynxB |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 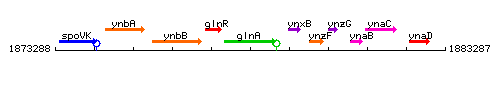
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
biosynthesis/ acquisition of amino acids, glutamate metabolism, transcription factors and their control, trigger enzyme, phosphoproteins, most abundant proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU17460
Phenotypes of a mutant
auxotrophic for glutamine
Database entries
- BsubCyc: BSU17460
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: ATP + L-glutamate + NH3 = ADP + phosphate + L-glutamine (according to Swiss-Prot)
- Protein family: glutamine synthetase family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information: K(M) for: Glu: 27 mM, ATP: 2.4 mM, ammonium: 0.18 mM; v(max): 3.7 µmol/min/mg
- Domains: glutamate binding flap (aa 300 ... 306: protects unstable intermediates from abberant hydrolysis)
- Modification:
- Cofactors: Mg(2+)
- Effectors of protein activity:
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- BsubCyc: BSU17460
- Structure:
- 4LNN (apo-GS) PubMed
- 3QAJ (complex with ATP)
- A general discussion of GS structure
- UniProt: P12425
- KEGG entry: [3]
- E.C. number: 6.3.1.2
Additional information
GlnA is a homooligomer of 12 subunits
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
- belongs to the 100 most abundant proteins PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium): 8140 PubMed
- number of protein molecules per cell (complex medium with amino acids, without glucose): 11334 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, exponential phase): 16645 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, early stationary phase after glucose exhaustion): 11115 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, late stationary phase after glucose exhaustion): 11598 PubMed
Biological materials
- Mutant:
- GP247 (glnA::cat), available in Jörg Stülke's lab
- BP148 (del(glnR-glnA)::cat), available in Fabian Commichau's lab
- GP1883 (del(glnR-glnA)::ermC), available in Fabian Commichau's and Jörg Stülke's labs
- Expression vector:
- expression/ purification from E. coli, with N-terminal Strep-tag (in pGP172): pGP174, available in Jörg Stülke's lab
- pGP177 (N-terminal Strep-tag, purification from B. subtilis, for SPINE, in pBQ200), available in Jörg Stülke's lab
- lacZ fusion: glnR-lacZ: pGP189 (in pAC7), available in Jörg Stülke's lab
- GFP fusion:
- two-hybrid system:
- Antibody: available in Karl Forchhammer's lab
Labs working on this gene/protein
Susan Fisher, Boston, USA homepage
Your additional remarks
References
Reviews
Original publications