Difference between revisions of "DnaA"
| Line 100: | Line 100: | ||
** [[DnaA]]-[[YabA]]-[[DnaN]] {{PubMed|12060778}} | ** [[DnaA]]-[[YabA]]-[[DnaN]] {{PubMed|12060778}} | ||
** [[DnaA]]-[[DnaD]] {{PubMed|22821970,11222620}} | ** [[DnaA]]-[[DnaD]] {{PubMed|22821970,11222620}} | ||
| − | ** [[SirA]]-[[DnaA]] {{PubMed|19682252,21239581}} | + | ** [[SirA]]-[[DnaA]] (N-terminal domain) {{PubMed|25041308,19682252,21239581}} |
** [[YqaH]]-[[DnaA]] {{PubMed|12060778}} | ** [[YqaH]]-[[DnaA]] {{PubMed|12060778}} | ||
** part of the [[primosome]]: [[DnaA]]-[[DnaG]]-[[DnaC]]-[[DnaI]]-[[DnaD]]-[[DnaB]] {{PubMed|22797751}} | ** part of the [[primosome]]: [[DnaA]]-[[DnaG]]-[[DnaC]]-[[DnaI]]-[[DnaD]]-[[DnaB]] {{PubMed|22797751}} | ||
| Line 169: | Line 169: | ||
<pubmed> 16120674, </pubmed> | <pubmed> 16120674, </pubmed> | ||
==Original publications== | ==Original publications== | ||
| − | <pubmed>18854156,19011033, 11222620,14651647,17140409 10844689 ,11222620 12060778 16461910 2987848 2168872 11207367, 19737352 19081080 17932079 19968790 19682252 20511500 21097613 21239581 21895792 22286949 22821970 23909787 22396664,21911367</pubmed> | + | <pubmed>18854156,19011033, 11222620,14651647,17140409 10844689 ,11222620 12060778 16461910 2987848 2168872 11207367, 19737352 19081080 17932079 19968790 19682252 20511500 21097613 21239581 21895792 22286949 22821970 23909787 22396664,21911367 25041308</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 11:36, 22 July 2014
- Description: AAA+ ATPase, replication initiation protein
| Gene name | dnaA |
| Synonyms | dnaH, dnaJ, dnaK |
| Essential | yes PubMed |
| Product | replication initiation protein |
| Function | DNA replication |
| Gene expression levels in SubtiExpress: dnaA | |
| Interactions involving this protein in SubtInteract: DnaA | |
| MW, pI | 50 kDa, 6.035 |
| Gene length, protein length | 1338 bp, 446 aa |
| Immediate neighbours | rpmH, dnaN |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed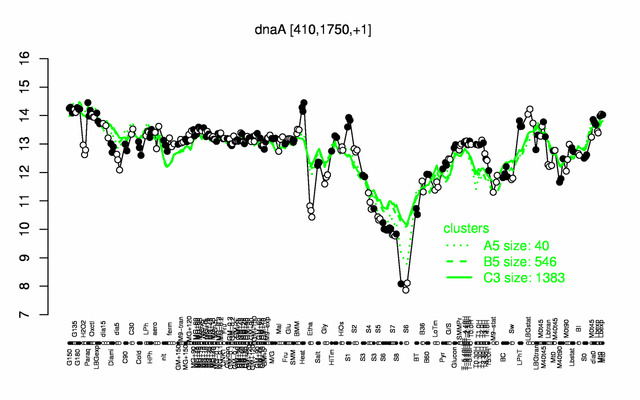
| |
Contents
Categories containing this gene/protein
DNA replication, essential genes
This gene is a member of the following regulons
The DnaA regulon
The gene
Basic information
- Locus tag: BSU00010
Phenotypes of a mutant
essential PubMed
Database entries
- BsubCyc: BSU00010
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- binds multiple regions in the oriC region, required for recruitment of proteins needed to load the replicative helicase DnaC
- Protein family: dnaA family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains: AAA+ domain
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- SirA displaces DnaA from the replication origin PubMed
- YabA inhibits co-operative binding of DnaA to the oriC DNA PubMed
- DnaA helix formation (and thus replication initiation) is inhibited by the interaction of either Soj, YabA or DnaN with the AAA+ domain of DnaA PubMed
- interaction with DnaD inhibits the ability of DnaA to cooperatively bind to DNA PubMed
- Interactions:
- DnaA assembles into a right-handed helical oligomer built upon interactions between neighbouring AAA+ domains PubMed
- Soj-DnaA PubMed
- DnaA-YabA PubMed
- DnaA-YabA-DnaN PubMed
- DnaA-DnaD PubMed
- SirA-DnaA (N-terminal domain) PubMed
- YqaH-DnaA PubMed
- part of the primosome: DnaA-DnaG-DnaC-DnaI-DnaD-DnaB PubMed
- Localization:
- throughout the cytoplasm PubMed
Database entries
- BsubCyc: BSU00010
- Structure:
- UniProt: P05648
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
- Philippe Noirot, Jouy-en-Josas, France homepage
- Peter Graumann, Freiburg University, Germany homepage
- Alan Grossman, MIT, Cambridge, MA, USA
- Heath Murray, Centre for Bacterial Cell Biology, Newcastle, UK homepage
Your additional remarks
References
Reviews
Geoffrey S Briggs, Wiep Klaas Smits, Panos Soultanas
Chromosomal replication initiation machinery of low-G+C-content Firmicutes.
J Bacteriol: 2012, 194(19);5162-70
[PubMed:22797751]
[WorldCat.org]
[DOI]
(I p)
An-Chun Chien, Norbert S Hill, Petra Anne Levin
Cell size control in bacteria.
Curr Biol: 2012, 22(9);R340-9
[PubMed:22575476]
[WorldCat.org]
[DOI]
(I p)
Alan C Leonard, Julia E Grimwade
Regulation of DnaA assembly and activity: taking directions from the genome.
Annu Rev Microbiol: 2011, 65;19-35
[PubMed:21639790]
[WorldCat.org]
[DOI]
(I p)
Alan C Leonard, Julia E Grimwade
Regulating DnaA complex assembly: it is time to fill the gaps.
Curr Opin Microbiol: 2010, 13(6);766-72
[PubMed:21035377]
[WorldCat.org]
[DOI]
(I p)
Tsutomu Katayama, Shogo Ozaki, Kenji Keyamura, Kazuyuki Fujimitsu
Regulation of the replication cycle: conserved and diverse regulatory systems for DnaA and oriC.
Nat Rev Microbiol: 2010, 8(3);163-70
[PubMed:20157337]
[WorldCat.org]
[DOI]
(I p)
The DnaA regulon
Original publications