Difference between revisions of "SsbB"
| Line 133: | Line 133: | ||
* '''Additional information:''' | * '''Additional information:''' | ||
| + | ** number of protein molecules per cell (minimal medium with glucose and ammonium): 144 {{PubMed|24696501}} | ||
=Biological materials = | =Biological materials = | ||
Revision as of 10:03, 17 April 2014
- Description: single-strand DNA-binding protein
| Gene name | ssbB |
| Synonyms | ywpH |
| Essential | no |
| Product | single-strand DNA-binding protein |
| Function | required for efficient genetic transformation |
| Gene expression levels in SubtiExpress: ssbB | |
| Interactions involving this protein in SubtInteract: SsbB | |
| MW, pI | 12 kDa, 6.876 |
| Gene length, protein length | 339 bp, 113 aa |
| Immediate neighbours | glcR, ywpG |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 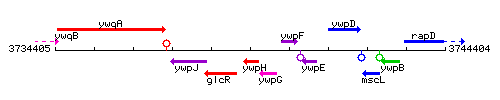
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed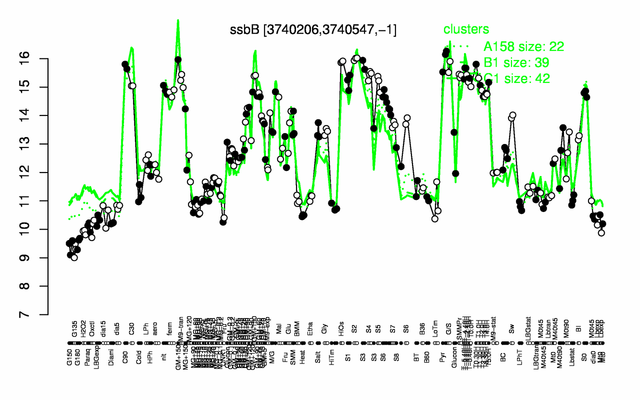
| |
Contents
Categories containing this gene/protein
DNA replication, genetic competence, DNA repair/ recombination, phosphoproteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU36310
Phenotypes of a mutant
Database entries
- BsubCyc: BSU36310
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- binds and protects internalized foreign ssDNA PubMed
- Protein family:
- Paralogous protein(s): SsbA
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity: phosphorylation strongly increases DNA-binding activity PubMed
Database entries
- BsubCyc: BSU36310
- UniProt: C0SPB6
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- expressed at the onset of stationary phase PubMed
- Additional information:
- number of protein molecules per cell (minimal medium with glucose and ammonium): 144 PubMed
Biological materials
- Mutant:
- Expression vector: for expression, purification in E. coli with N-terminal His-tag, pRSETA available in Gerth lab
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Justin S Lenhart, Jeremy W Schroeder, Brian W Walsh, Lyle A Simmons
DNA repair and genome maintenance in Bacillus subtilis.
Microbiol Mol Biol Rev: 2012, 76(3);530-64
[PubMed:22933559]
[WorldCat.org]
[DOI]
(I p)
Original publications
Tribhuwan Yadav, Begoña Carrasco, James Hejna, Yuki Suzuki, Kunio Takeyasu, Juan C Alonso
Bacillus subtilis DprA recruits RecA onto single-stranded DNA and mediates annealing of complementary strands coated by SsbB and SsbA.
J Biol Chem: 2013, 288(31);22437-50
[PubMed:23779106]
[WorldCat.org]
[DOI]
(I p)
Aimee H Marceau, Douglas A Bernstein, Brian W Walsh, Walker Shapiro, Lyle A Simmons, James L Keck
Protein interactions in genome maintenance as novel antibacterial targets.
PLoS One: 2013, 8(3);e58765
[PubMed:23536821]
[WorldCat.org]
[DOI]
(I p)
Alexander K W Elsholz, Kürsad Turgay, Stephan Michalik, Bernd Hessling, Katrin Gronau, Dan Oertel, Ulrike Mäder, Jörg Bernhardt, Dörte Becher, Michael Hecker, Ulf Gerth
Global impact of protein arginine phosphorylation on the physiology of Bacillus subtilis.
Proc Natl Acad Sci U S A: 2012, 109(19);7451-6
[PubMed:22517742]
[WorldCat.org]
[DOI]
(I p)
Pierre Nicolas, Ulrike Mäder, Etienne Dervyn, Tatiana Rochat, Aurélie Leduc, Nathalie Pigeonneau, Elena Bidnenko, Elodie Marchadier, Mark Hoebeke, Stéphane Aymerich, Dörte Becher, Paola Bisicchia, Eric Botella, Olivier Delumeau, Geoff Doherty, Emma L Denham, Mark J Fogg, Vincent Fromion, Anne Goelzer, Annette Hansen, Elisabeth Härtig, Colin R Harwood, Georg Homuth, Hanne Jarmer, Matthieu Jules, Edda Klipp, Ludovic Le Chat, François Lecointe, Peter Lewis, Wolfram Liebermeister, Anika March, Ruben A T Mars, Priyanka Nannapaneni, David Noone, Susanne Pohl, Bernd Rinn, Frank Rügheimer, Praveen K Sappa, Franck Samson, Marc Schaffer, Benno Schwikowski, Leif Steil, Jörg Stülke, Thomas Wiegert, Kevin M Devine, Anthony J Wilkinson, Jan Maarten van Dijl, Michael Hecker, Uwe Völker, Philippe Bessières, Philippe Noirot
Condition-dependent transcriptome reveals high-level regulatory architecture in Bacillus subtilis.
Science: 2012, 335(6072);1103-6
[PubMed:22383849]
[WorldCat.org]
[DOI]
(I p)
Tribhuwan Yadav, Begoña Carrasco, Angela R Myers, Nicholas P George, James L Keck, Juan C Alonso
Genetic recombination in Bacillus subtilis: a division of labor between two single-strand DNA-binding proteins.
Nucleic Acids Res: 2012, 40(12);5546-59
[PubMed:22373918]
[WorldCat.org]
[DOI]
(I p)
Naomi Kramer, Jeanette Hahn, David Dubnau
Multiple interactions among the competence proteins of Bacillus subtilis.
Mol Microbiol: 2007, 65(2);454-64
[PubMed:17630974]
[WorldCat.org]
[DOI]
(P p)
Ivan Mijakovic, Dina Petranovic, Boris Macek, Tina Cepo, Matthias Mann, Julian Davies, Peter R Jensen, Dusica Vujaklija
Bacterial single-stranded DNA-binding proteins are phosphorylated on tyrosine.
Nucleic Acids Res: 2006, 34(5);1588-96
[PubMed:16549871]
[WorldCat.org]
[DOI]
(I e)
Jeanette Hahn, Berenike Maier, Bert Jan Haijema, Michael Sheetz, David Dubnau
Transformation proteins and DNA uptake localize to the cell poles in Bacillus subtilis.
Cell: 2005, 122(1);59-71
[PubMed:16009133]
[WorldCat.org]
[DOI]
(P p)
Cordula Lindner, Reindert Nijland, Mariska van Hartskamp, Sierd Bron, Leendert W Hamoen, Oscar P Kuipers
Differential expression of two paralogous genes of Bacillus subtilis encoding single-stranded DNA binding protein.
J Bacteriol: 2004, 186(4);1097-105
[PubMed:14762004]
[WorldCat.org]
[DOI]
(P p)
Mitsuo Ogura, Hirotake Yamaguchi, Kazuo Kobayashi, Naotake Ogasawara, Yasutaro Fujita, Teruo Tanaka
Whole-genome analysis of genes regulated by the Bacillus subtilis competence transcription factor ComK.
J Bacteriol: 2002, 184(9);2344-51
[PubMed:11948146]
[WorldCat.org]
[DOI]
(P p)