Difference between revisions of "GcaD"
(→Biological materials) |
|||
| Line 63: | Line 63: | ||
=== Database entries === | === Database entries === | ||
| + | * '''BsubCyc:''' [http://bsubcyc.org/BSUB/NEW-IMAGE?type=NIL&object=BSU00500&redirect=T BSU00500] | ||
* '''DBTBS entry:''' [http://dbtbs.hgc.jp/COG/prom/gcaD-prs-ctc.html] | * '''DBTBS entry:''' [http://dbtbs.hgc.jp/COG/prom/gcaD-prs-ctc.html] | ||
| Line 101: | Line 102: | ||
=== Database entries === | === Database entries === | ||
| + | * '''BsubCyc:''' [http://bsubcyc.org/BSUB/NEW-IMAGE?type=NIL&object=BSU00500&redirect=T BSU00500] | ||
* '''Structure:''' [http://www.pdb.org/pdb/explore/explore.do?structureId=1hm9 1HM9] (from ''Streptococcus pneumoniae'', 49% identity) {{PubMed|11118459}} | * '''Structure:''' [http://www.pdb.org/pdb/explore/explore.do?structureId=1hm9 1HM9] (from ''Streptococcus pneumoniae'', 49% identity) {{PubMed|11118459}} | ||
Revision as of 12:46, 2 April 2014
- Description: bifunctional N-acetylglucosamine-1-phosphate uridyltransferase/glucosamine-1-phosphate acetyltransferase
| Gene name | gcaD |
| Synonyms | tms, tms26 |
| Essential | yes PubMed |
| Product | bifunctional N-acetylglucosamine-1-phosphate
uridyltransferase/glucosamine-1-phosphate acetyltransferase |
| Function | cell wall metabolism |
| Gene expression levels in SubtiExpress: gcaD | |
| Metabolic function and regulation of this protein in SubtiPathways: gcaD | |
| MW, pI | 49 kDa, 5.65 |
| Gene length, protein length | 1368 bp, 456 aa |
| Immediate neighbours | spoVG, prs |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed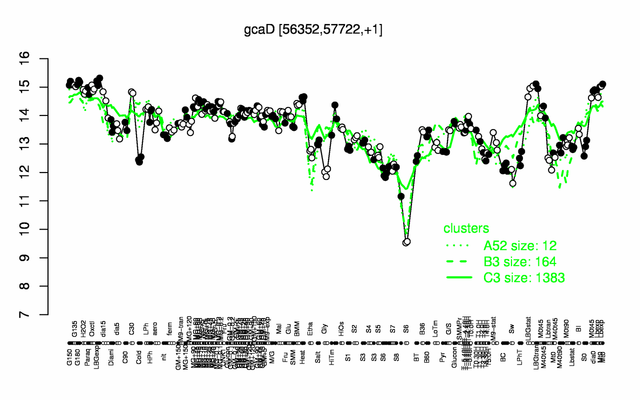
| |
Contents
Categories containing this gene/protein
cell wall synthesis, biosynthesis of cell wall components, essential genes
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU00500
Phenotypes of a mutant
essential PubMed
Database entries
- BsubCyc: BSU00500
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Acetyl-CoA + alpha-D-glucosamine 1-phosphate = CoA + N-acetyl-alpha-D-glucosamine 1-phosphate (according to Swiss-Prot)
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization:
- cytoplasm (according to Swiss-Prot)
Database entries
- BsubCyc: BSU00500
- UniProt: P14192
- KEGG entry: [3]
- E.C. number: 2.7.7.23
Additional information
Expression and regulation
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- pGP2596: (IPTG inducible expression, purification in E. coli with N-terminal His-tag, in pWH844), available in Jörg Stülke's lab
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References