Difference between revisions of "YraA"
| Line 1: | Line 1: | ||
| − | * '''Description:''' general stress protein, degradation of damaged thiol-containing proteins, | + | * '''Description:''' general stress protein, degradation of damaged thiol-containing proteins, glyoxalase III-like enzyme <br/><br/> |
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
| Line 10: | Line 10: | ||
|style="background:#ABCDEF;" align="center"| '''Essential''' || no | |style="background:#ABCDEF;" align="center"| '''Essential''' || no | ||
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Product''' || | + | |style="background:#ABCDEF;" align="center"| '''Product''' || glyoxalase III-like enzyme |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"|'''Function''' || | + | |style="background:#ABCDEF;" align="center"|'''Function''' || detoxification of methylglyoxal |
|- | |- | ||
|colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http://subtiwiki.uni-goettingen.de/apps/expression/ ''Subti''Express]''': [http://subtiwiki.uni-goettingen.de/apps/expression/expression.php?search=BSU27020 yraA] | |colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http://subtiwiki.uni-goettingen.de/apps/expression/ ''Subti''Express]''': [http://subtiwiki.uni-goettingen.de/apps/expression/expression.php?search=BSU27020 yraA] | ||
| Line 64: | Line 64: | ||
=== Additional information=== | === Additional information=== | ||
| − | |||
| − | |||
| − | |||
=The protein= | =The protein= | ||
| Line 82: | Line 79: | ||
* '''Kinetic information:''' | * '''Kinetic information:''' | ||
| − | * '''Domains:''' | + | * '''[[Domains]]:''' |
* '''Modification:''' | * '''Modification:''' | ||
| − | * ''' | + | * '''[[Cofactors]]:''' |
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| Line 146: | Line 143: | ||
=References= | =References= | ||
| − | <pubmed>17434969,19170879,22582280,15805528 17434969, </pubmed> | + | <pubmed>17434969,19170879,22582280,15805528 17434969, 24330391 </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 18:12, 7 January 2014
- Description: general stress protein, degradation of damaged thiol-containing proteins, glyoxalase III-like enzyme
| Gene name | yraA |
| Synonyms | |
| Essential | no |
| Product | glyoxalase III-like enzyme |
| Function | detoxification of methylglyoxal |
| Gene expression levels in SubtiExpress: yraA | |
| MW, pI | 18 kDa, 4.729 |
| Gene length, protein length | 507 bp, 169 aa |
| Immediate neighbours | adhA, sacC |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 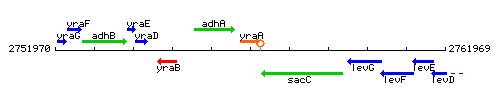
This image was kindly provided by SubtiList
| |
| Expression at a glance PubMed 500px | |
Contents
Categories containing this gene/protein
proteolysis, general stress proteins (controlled by SigB), resistance against oxidative and electrophile stress
This gene is a member of the following regulons
AdhR regulon, SigB regulon, SigM regulon
The gene
Basic information
- Locus tag: BSU27020
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: peptidase C56 family (according to Swiss-Prot)
- Paralogous protein(s): YfkM
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity:
Database entries
- Structure:
- UniProt: O06006
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Pete Chandrangsu, Renata Dusi, Chris J Hamilton, John D Helmann
Methylglyoxal resistance in Bacillus subtilis: contributions of bacillithiol-dependent and independent pathways.
Mol Microbiol: 2014, 91(4);706-15
[PubMed:24330391]
[WorldCat.org]
[DOI]
(I p)
Alexander Reder, Dirk Höper, Ulf Gerth, Michael Hecker
Contributions of individual σB-dependent general stress genes to oxidative stress resistance of Bacillus subtilis.
J Bacteriol: 2012, 194(14);3601-10
[PubMed:22582280]
[WorldCat.org]
[DOI]
(I p)
Thi Thu Huyen Nguyen, Warawan Eiamphungporn, Ulrike Mäder, Manuel Liebeke, Michael Lalk, Michael Hecker, John D Helmann, Haike Antelmann
Genome-wide responses to carbonyl electrophiles in Bacillus subtilis: control of the thiol-dependent formaldehyde dehydrogenase AdhA and cysteine proteinase YraA by the MerR-family regulator YraB (AdhR).
Mol Microbiol: 2009, 71(4);876-94
[PubMed:19170879]
[WorldCat.org]
[DOI]
(I p)
Adrian J Jervis, Penny D Thackray, Chris W Houston, Malcolm J Horsburgh, Anne Moir
SigM-responsive genes of Bacillus subtilis and their promoters.
J Bacteriol: 2007, 189(12);4534-8
[PubMed:17434969]
[WorldCat.org]
[DOI]
(P p)
Dirk Höper, Uwe Völker, Michael Hecker
Comprehensive characterization of the contribution of individual SigB-dependent general stress genes to stress resistance of Bacillus subtilis.
J Bacteriol: 2005, 187(8);2810-26
[PubMed:15805528]
[WorldCat.org]
[DOI]
(P p)