Difference between revisions of "PycA"
(→Biological materials) |
|||
| Line 83: | Line 83: | ||
* '''Modification:''' | * '''Modification:''' | ||
| − | * ''' | + | * '''[[Cofactors]]:''' biotin |
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
Revision as of 09:00, 25 November 2013
- Description: pyruvate carboxylase
| Gene name | pycA |
| Synonyms | ylaP |
| Essential | no |
| Product | pyruvate carboxylase |
| Function | replenishment of the oxaloacetate pool |
| Gene expression levels in SubtiExpress: pycA | |
| Metabolic function and regulation of this protein in SubtiPathways: Central C-metabolism, Biotin | |
| MW, pI | 127 kDa, 5.407 |
| Gene length, protein length | 3444 bp, 1148 aa |
| Immediate neighbours | ftsW, ctaA |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 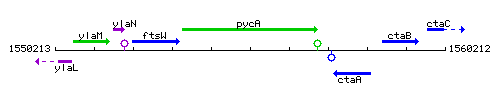
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed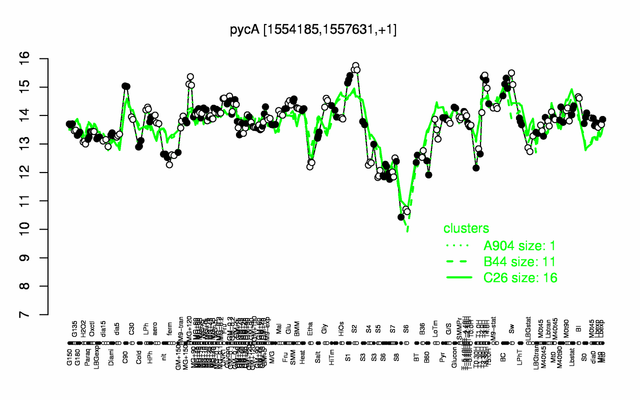
| |
Contents
Categories containing this gene/protein
carbon core metabolism, membrane proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU14860
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactors: biotin
- Effectors of protein activity:
- Localization: membrane associated PubMed
Database entries
- Structure: 3BG5 (S. aureus)
- UniProt: Q9KWU4
- KEGG entry: [3]
- E.C. number: 6.4.1.1
Additional information
PycA binds to StrepTactin, and may be co-purified when purifying Strep-tagged proteins by SPINE.
PycA is subject to Clp-dependent proteolysis upon glucose starvation PubMed
Expression and regulation
- Regulation:
- subject to positive stringent control upon lysine starvation PubMed
- Regulatory mechanism:
- stringent response: due to presence of adenines at +1 and +2 positions of the transcript PubMed
- Additional information: subject to Clp-dependent proteolysis upon glucose starvation PubMed
Biological materials
- Mutant: GP793 (cat), available in Jörg Stülke's lab
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Sarawut Jitrapakdee, Martin St Maurice, Ivan Rayment, W Wallace Cleland, John C Wallace, Paul V Attwood
Structure, mechanism and regulation of pyruvate carboxylase.
Biochem J: 2008, 413(3);369-87
[PubMed:18613815]
[WorldCat.org]
[DOI]
(I p)
Sarawut Jitrapakdee, John C Wallace
The biotin enzyme family: conserved structural motifs and domain rearrangements.
Curr Protein Pept Sci: 2003, 4(3);217-29
[PubMed:12769720]
[WorldCat.org]
[DOI]
(P p)
S Jitrapakdee, J C Wallace
Structure, function and regulation of pyruvate carboxylase.
Biochem J: 1999, 340 ( Pt 1)(Pt 1);1-16
[PubMed:10229653]
[WorldCat.org]
[DOI]
(P p)
J C Wallace, S Jitrapakdee, A Chapman-Smith
Pyruvate carboxylase.
Int J Biochem Cell Biol: 1998, 30(1);1-5
[PubMed:9597748]
[WorldCat.org]
[DOI]
(P p)
P V Attwood
The structure and the mechanism of action of pyruvate carboxylase.
Int J Biochem Cell Biol: 1995, 27(3);231-49
[PubMed:7780827]
[WorldCat.org]
[DOI]
(P p)
Original publications