Difference between revisions of "HutP"
(→Original publications) |
|||
| Line 36: | Line 36: | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
| − | <br/><br/><br/><br/><br/><br/> | + | <br/><br/><br/><br/> |
| + | <br/><br/> | ||
= [[Categories]] containing this gene/protein = | = [[Categories]] containing this gene/protein = | ||
| Line 87: | Line 88: | ||
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| + | ** HutP binds its RNA target to cause antitermination in the presence of histidine {{PubMed|23748184}} | ||
* '''[[SubtInteract|Interactions]]:''' | * '''[[SubtInteract|Interactions]]:''' | ||
| Line 109: | Line 111: | ||
* '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=hutP_4041483_4041938_1 hutP] {{PubMed|22383849}} | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=hutP_4041483_4041938_1 hutP] {{PubMed|22383849}} | ||
| − | * '''Sigma factor:''' [[SigA]] {{PubMed|8071225}} | + | * '''[[Sigma factor]]:''' [[SigA]] {{PubMed|8071225}} |
* '''Regulation:''' | * '''Regulation:''' | ||
| Line 144: | Line 146: | ||
==Original publications== | ==Original publications== | ||
| + | '''Additional publications:''' {{PubMed|23748184}} | ||
<pubmed>12903241,8682780, 8071225 10217782 18083814 22900538 23748184 </pubmed> | <pubmed>12903241,8682780, 8071225 10217782 18083814 22900538 23748184 </pubmed> | ||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 08:32, 12 June 2013
- Description: transcriptional antiterminator of the hut operon
| Gene name | hutP |
| Synonyms | hutP1 |
| Essential | no |
| Product | transcriptional antiterminator |
| Function | regulation of histidine utilization |
| Gene expression levels in SubtiExpress: hutP | |
| Metabolic function and regulation of this protein in SubtiPathways: His | |
| MW, pI | 16 kDa, 6.057 |
| Gene length, protein length | 453 bp, 151 aa |
| Immediate neighbours | yxzL, hutH |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 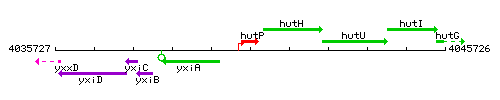
This image was kindly provided by SubtiList
| |
| Expression at a glance PubMed 500px | |
Contents
Categories containing this gene/protein
utilization of amino acids, transcription factors and their control, RNA binding regulators
This gene is a member of the following regulons
The HutP regulon: hutH-hutU-hutI-hutG-hutM
The gene
Basic information
- Locus tag: BSU39340
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: hutP family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- HutP binds its RNA target to cause antitermination in the presence of histidine PubMed
Database entries
- UniProt: P10943
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Original publications
Additional publications: PubMed
Balasundaresan Dhakshnamoorthy, Hiroshi Mizuno, Penmetcha K R Kumar
Alternative binding modes of l-histidine guided by metal ions for the activation of the antiterminator protein HutP of Bacillus subtilis.
J Struct Biol: 2013, 183(3);512-518
[PubMed:23748184]
[WorldCat.org]
[DOI]
(I p)
Bogumiła C Marciniak, Monika Pabijaniak, Anne de Jong, Robert Dűhring, Gerald Seidel, Wolfgang Hillen, Oscar P Kuipers
High- and low-affinity cre boxes for CcpA binding in Bacillus subtilis revealed by genome-wide analysis.
BMC Genomics: 2012, 13;401
[PubMed:22900538]
[WorldCat.org]
[DOI]
(I e)
Boris R Belitsky, Abraham L Sonenshein
Genetic and biochemical analysis of CodY-binding sites in Bacillus subtilis.
J Bacteriol: 2008, 190(4);1224-36
[PubMed:18083814]
[WorldCat.org]
[DOI]
(I p)
M Oda, N Kobayashi, Y Kurusu, M Fujita
Analysis of histidine-dependent antitermination in Bacillus subtilis hut operon.
Nucleic Acids Symp Ser: 2000, (44);5-6
[PubMed:12903241]
[WorldCat.org]
[DOI]
(P p)
J M Zalieckas, L V Wray, S H Fisher
trans-acting factors affecting carbon catabolite repression of the hut operon in Bacillus subtilis.
J Bacteriol: 1999, 181(9);2883-8
[PubMed:10217782]
[WorldCat.org]
[DOI]
(P p)
S H Fisher, K Rohrer, A E Ferson
Role of CodY in regulation of the Bacillus subtilis hut operon.
J Bacteriol: 1996, 178(13);3779-84
[PubMed:8682780]
[WorldCat.org]
[DOI]
(P p)
L V Wray, S H Fisher
Analysis of Bacillus subtilis hut operon expression indicates that histidine-dependent induction is mediated primarily by transcriptional antitermination and that amino acid repression is mediated by two mechanisms: regulation of transcription initiation and inhibition of histidine transport.
J Bacteriol: 1994, 176(17);5466-73
[PubMed:8071225]
[WorldCat.org]
[DOI]
(P p)