Difference between revisions of "YumC"
(→Database entries) |
|||
| Line 103: | Line 103: | ||
* '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu:BSU32110] | * '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu:BSU32110] | ||
| − | * '''E.C. number:''' | + | * '''E.C. number:''' [http://www.expasy.org/enzyme/1.18.1.2 1.18.1.2] |
=== Additional information=== | === Additional information=== | ||
Revision as of 18:37, 18 February 2014
- Description: ferredoxin-NAD(P)+ oxidoreductase
| Gene name | yumC |
| Synonyms | |
| Essential | yes PubMed |
| Product | ferredoxin-NAD(P)+ oxidoreductase |
| Function | redox reactions that involve ferredoxin |
| Gene expression levels in SubtiExpress: yumC | |
| MW, pI | 36 kDa, 5.573 |
| Gene length, protein length | 996 bp, 332 aa |
| Immediate neighbours | yumB, yuzG |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 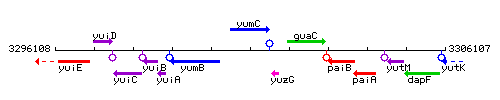
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed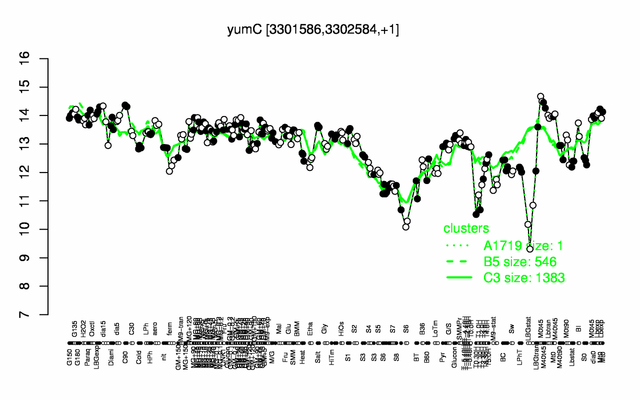
| |
Contents
Categories containing this gene/protein
electron transport/ other, essential genes
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU32110
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: 2 reduced ferredoxin + NADP+ + H+ = 2 oxidized ferredoxin + NADPH (according to Swiss-Prot)
- Protein family: ferredoxin--NADP reductase type 2 family (according to Swiss-Prot)http://www.ncbi.nlm.nih.gov/pubmed
- Paralogous protein(s): YcgT
Extended information on the protein
- Kinetic information:
- Domains:
- Modification: active site Cys85 is S-bacillithiolated by NaOCl stress in B. subtilis and other Bacillus speciesPubMed
- Cofactor(s): FAD, NADPH PubMed
- Effectors of protein activity:
- Interactions: dimeric protein PubMed
- Localization:
Database entries
- UniProt: O05268
- KEGG entry: [2]
- E.C. number: 1.18.1.2
Additional information
Expression and regulation
- Operon:
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References