Difference between revisions of "DnaG"
| Line 103: | Line 103: | ||
=== Database entries === | === Database entries === | ||
| − | * '''Structure:''' [http://www.rcsb.org/pdb/explore.do?structureId=1D0Q 1D0Q] (zinc binding domain, Geobacillus stearothermophilus) | + | * '''Structure:''' |
| + | ** [http://www.rcsb.org/pdb/explore.do?structureId=1D0Q 1D0Q] (zinc binding domain, Geobacillus stearothermophilus) | ||
| + | ** [http://www.rcsb.org/pdb/explore.do?structureId=1Z8S 1Z8S] (DnaB binding domain, AA 452-597, Geobacillus stearothermophilus) | ||
| + | ** [http://www.rcsb.org/pdb/explore.do?structureId=4M4W 4M4W] (the [[DnaC]]-[[DnaI]]-[[DnaG]] complex) {{PubMed|24048025}} | ||
* '''UniProt:''' [http://www.uniprot.org/uniprot/P05096 P05096] | * '''UniProt:''' [http://www.uniprot.org/uniprot/P05096 P05096] | ||
| Line 154: | Line 157: | ||
==Original publications== | ==Original publications== | ||
| − | <pubmed>3919021, 20122408,14651647, 16479537, 19415803 17350574 23563155 23268446,21419346</pubmed> | + | <pubmed>3919021, 20122408,14651647, 16479537, 19415803 17350574 23563155 23268446,21419346 24048025</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 08:32, 23 September 2013
- Description: DNA primase, part of the replisome
| Gene name | dnaG |
| Synonyms | dnaE |
| Essential | yes PubMed |
| Product | DNA primase |
| Function | DNA replication |
| Gene expression levels in SubtiExpress: dnaG | |
| Interactions involving this protein in SubtInteract: DnaG | |
| MW, pI | 68 kDa, 6.706 |
| Gene length, protein length | 1809 bp, 603 aa |
| Immediate neighbours | sigA, antE |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 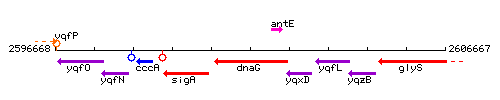
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed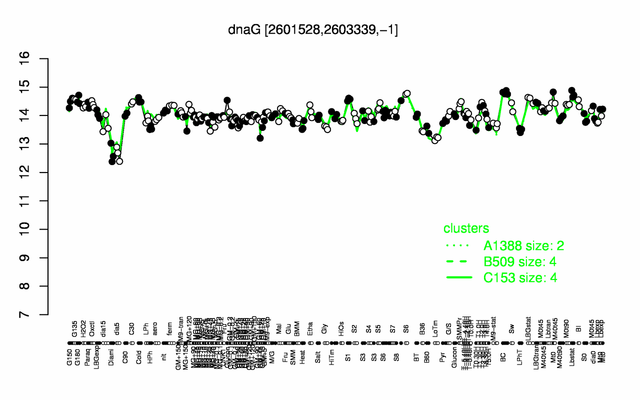
| |
Contents
Categories containing this gene/protein
DNA replication, essential genes
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU25210
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- required for bacteriophage SPP1 replication PubMed
- Protein family: DNA primase family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: Cytoplasm (Homogeneous) PubMed
Database entries
- Structure:
- UniProt: P05096
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Additional information:
- enzymatic activity is inhibited by (p)ppGpp during the ´stringent response´ PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Original publications
Bin Liu, William K Eliason, Thomas A Steitz
Structure of a helicase-helicase loader complex reveals insights into the mechanism of bacterial primosome assembly.
Nat Commun: 2013, 4;2495
[PubMed:24048025]
[WorldCat.org]
[DOI]
(I p)
Olivier Rannou, Emmanuelle Le Chatelier, Marilynn A Larson, Hamid Nouri, Bérengère Dalmais, Charles Laughton, Laurent Jannière, Panos Soultanas
Functional interplay of DnaE polymerase, DnaG primase and DnaC helicase within a ternary complex, and primase to polymerase hand-off during lagging strand DNA replication in Bacillus subtilis.
Nucleic Acids Res: 2013, 41(10);5303-20
[PubMed:23563155]
[WorldCat.org]
[DOI]
(I p)
Elena M Seco, John C Zinder, Carol M Manhart, Ambra Lo Piano, Charles S McHenry, Silvia Ayora
Bacteriophage SPP1 DNA replication strategies promote viral and disable host replication in vitro.
Nucleic Acids Res: 2013, 41(3);1711-21
[PubMed:23268446]
[WorldCat.org]
[DOI]
(I p)
Masayuki Su'etsugu, Jeff Errington
The replicase sliding clamp dynamically accumulates behind progressing replication forks in Bacillus subtilis cells.
Mol Cell: 2011, 41(6);720-32
[PubMed:21419346]
[WorldCat.org]
[DOI]
(I p)
Glenn M Sanders, H Garry Dallmann, Charles S McHenry
Reconstitution of the B. subtilis replisome with 13 proteins including two distinct replicases.
Mol Cell: 2010, 37(2);273-81
[PubMed:20122408]
[WorldCat.org]
[DOI]
(I p)
Kiran Chintakayala, Cristina Machón, Anna Haroniti, Marilyn A Larson, Steven H Hinrichs, Mark A Griep, Panos Soultanas
Allosteric regulation of the primase (DnaG) activity by the clamp-loader (tau) in vitro.
Mol Microbiol: 2009, 72(2);537-49
[PubMed:19415803]
[WorldCat.org]
[DOI]
(I p)
Jue D Wang, Glenn M Sanders, Alan D Grossman
Nutritional control of elongation of DNA replication by (p)ppGpp.
Cell: 2007, 128(5);865-75
[PubMed:17350574]
[WorldCat.org]
[DOI]
(P p)
Jean-Christophe Meile, Ling Juan Wu, S Dusko Ehrlich, Jeff Errington, Philippe Noirot
Systematic localisation of proteins fused to the green fluorescent protein in Bacillus subtilis: identification of new proteins at the DNA replication factory.
Proteomics: 2006, 6(7);2135-46
[PubMed:16479537]
[WorldCat.org]
[DOI]
(P p)
Virginie Molle, Masaya Fujita, Shane T Jensen, Patrick Eichenberger, José E González-Pastor, Jun S Liu, Richard Losick
The Spo0A regulon of Bacillus subtilis.
Mol Microbiol: 2003, 50(5);1683-701
[PubMed:14651647]
[WorldCat.org]
[DOI]
(P p)
L F Wang, C W Price, R H Doi
Bacillus subtilis dnaE encodes a protein homologous to DNA primase of Escherichia coli.
J Biol Chem: 1985, 260(6);3368-72
[PubMed:3919021]
[WorldCat.org]
(P p)