Difference between revisions of "TatAD"
| Line 16: | Line 16: | ||
|colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http://subtiwiki.uni-goettingen.de/apps/expression/ ''Subti''Express]''': [http://subtiwiki.uni-goettingen.de/apps/expression/expression.php?search=BSU02630 tatAD] | |colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http://subtiwiki.uni-goettingen.de/apps/expression/ ''Subti''Express]''': [http://subtiwiki.uni-goettingen.de/apps/expression/expression.php?search=BSU02630 tatAD] | ||
|- | |- | ||
| − | |colspan="2" style="background:#FAF8CC;" align="center"| '''Interactions involving this protein in [http:// | + | |colspan="2" style="background:#FAF8CC;" align="center"| '''Interactions involving this protein in [http://subtiwiki.uni-goettingen.de/interact/ ''Subt''Interact]''': [http://subtiwiki.uni-goettingen.de/interact/index.php?protein=TatAD TatAD] |
|- | |- | ||
|colspan="2" style="background:#FAF8CC;" align="center"| '''Metabolic function and regulation of this protein in [[SubtiPathways|''Subti''Pathways]]: <br/>[http://subtiwiki.uni-goettingen.de/wiki/index.php/Protein_secretion Protein secretion]''' | |colspan="2" style="background:#FAF8CC;" align="center"| '''Metabolic function and regulation of this protein in [[SubtiPathways|''Subti''Pathways]]: <br/>[http://subtiwiki.uni-goettingen.de/wiki/index.php/Protein_secretion Protein secretion]''' | ||
Revision as of 15:07, 11 November 2013
- Description: component of the TatAD-TatCD twin-arginine translocase
| Gene name | tatAD |
| Synonyms | yczB |
| Essential | no |
| Product | component of the twin-arginine translocation pathway |
| Function | TAT protein secretion |
| Gene expression levels in SubtiExpress: tatAD | |
| Interactions involving this protein in SubtInteract: TatAD | |
| Metabolic function and regulation of this protein in SubtiPathways: Protein secretion | |
| MW, pI | 7 kDa, 9.693 |
| Gene length, protein length | 210 bp, 70 aa |
| Immediate neighbours | phoD, tatCD |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 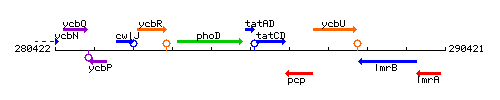
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed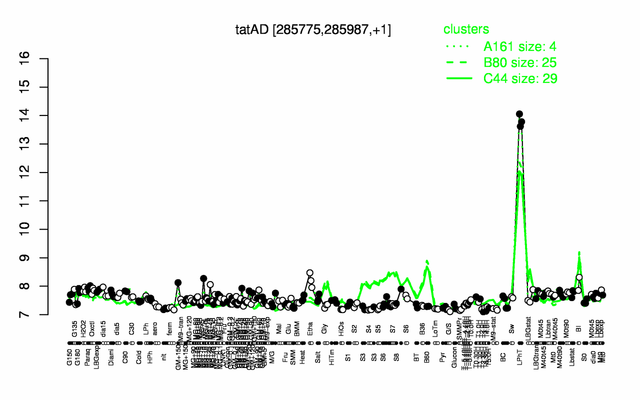
| |
Contents
Categories containing this gene/protein
phosphate metabolism, protein secretion, membrane proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU02630
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Protein family: tatA/E family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: membrane (according to Swiss-Prot), forms foci at the division sites and cell poles, localization requires interaction with TatCD or TatCY PubMed
Database entries
- UniProt: O31467
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Oscar Kuipers, University of Groningen, The Netherlands Homepage
Your additional remarks
References
Reviews
Original publications
Daniel Beck, Nishi Vasisht, Jacopo Baglieri, Carmine G Monteferrante, Jan Maarten van Dijl, Colin Robinson, Corinne J Smith
Ultrastructural characterisation of Bacillus subtilis TatA complexes suggests they are too small to form homooligomeric translocation pores.
Biochim Biophys Acta: 2013, 1833(8);1811-9
[PubMed:23567937]
[WorldCat.org]
[DOI]
(P p)
Carmine G Monteferrante, Calum MacKichan, Elodie Marchadier, Maria-Victoria Prejean, Rut Carballido-López, Jan Maarten van Dijl
Mapping the twin-arginine protein translocation network of Bacillus subtilis.
Proteomics: 2013, 13(5);800-11
[PubMed:23180473]
[WorldCat.org]
[DOI]
(I p)
Pierre Nicolas, Ulrike Mäder, Etienne Dervyn, Tatiana Rochat, Aurélie Leduc, Nathalie Pigeonneau, Elena Bidnenko, Elodie Marchadier, Mark Hoebeke, Stéphane Aymerich, Dörte Becher, Paola Bisicchia, Eric Botella, Olivier Delumeau, Geoff Doherty, Emma L Denham, Mark J Fogg, Vincent Fromion, Anne Goelzer, Annette Hansen, Elisabeth Härtig, Colin R Harwood, Georg Homuth, Hanne Jarmer, Matthieu Jules, Edda Klipp, Ludovic Le Chat, François Lecointe, Peter Lewis, Wolfram Liebermeister, Anika March, Ruben A T Mars, Priyanka Nannapaneni, David Noone, Susanne Pohl, Bernd Rinn, Frank Rügheimer, Praveen K Sappa, Franck Samson, Marc Schaffer, Benno Schwikowski, Leif Steil, Jörg Stülke, Thomas Wiegert, Kevin M Devine, Anthony J Wilkinson, Jan Maarten van Dijl, Michael Hecker, Uwe Völker, Philippe Bessières, Philippe Noirot
Condition-dependent transcriptome reveals high-level regulatory architecture in Bacillus subtilis.
Science: 2012, 335(6072);1103-6
[PubMed:22383849]
[WorldCat.org]
[DOI]
(I p)
James P Barnett, Janna Lawrence, Sharon Mendel, Colin Robinson
Expression of the bifunctional Bacillus subtilis TatAd protein in Escherichia coli reveals distinct TatA/B-family and TatB-specific domains.
Arch Microbiol: 2011, 193(8);583-94
[PubMed:21479530]
[WorldCat.org]
[DOI]
(I p)
René van der Ploeg, Ulrike Mäder, Georg Homuth, Marc Schaffer, Emma L Denham, Carmine G Monteferrante, Marcus Miethke, Mohamed A Marahiel, Colin R Harwood, Theresa Winter, Michael Hecker, Haike Antelmann, Jan Maarten van Dijl
Environmental salinity determines the specificity and need for Tat-dependent secretion of the YwbN protein in Bacillus subtilis.
PLoS One: 2011, 6(3);e18140
[PubMed:21479178]
[WorldCat.org]
[DOI]
(I e)
Torsten H Walther, Stephan L Grage, Nadine Roth, Anne S Ulrich
Membrane alignment of the pore-forming component TatA(d) of the twin-arginine translocase from Bacillus subtilis resolved by solid-state NMR spectroscopy.
J Am Chem Soc: 2010, 132(45);15945-56
[PubMed:20977272]
[WorldCat.org]
[DOI]
(I p)
Yunfei Hu, Enwei Zhao, Hongwei Li, Bin Xia, Changwen Jin
Solution NMR structure of the TatA component of the twin-arginine protein transport system from gram-positive bacterium Bacillus subtilis.
J Am Chem Soc: 2010, 132(45);15942-4
[PubMed:20726548]
[WorldCat.org]
[DOI]
(I p)
Anja N J A Ridder, Esther J de Jong, Jan D H Jongbloed, Oscar P Kuipers
Subcellular localization of TatAd of Bacillus subtilis depends on the presence of TatCd or TatCy.
J Bacteriol: 2009, 191(13);4410-8
[PubMed:19395490]
[WorldCat.org]
[DOI]
(I p)
Jan D H Jongbloed, Ulrike Grieger, Haike Antelmann, Michael Hecker, Reindert Nijland, Sierd Bron, Jan Maarten van Dijl
Two minimal Tat translocases in Bacillus.
Mol Microbiol: 2004, 54(5);1319-25
[PubMed:15554971]
[WorldCat.org]
[DOI]
(P p)
Ovidiu I Pop, Martin Westermann, Rudolf Volkmer-Engert, Daniela Schulz, Cornelius Lemke, Sandra Schreiber, Roman Gerlach, Reinhard Wetzker, Jörg P Müller
Sequence-specific binding of prePhoD to soluble TatAd indicates protein-mediated targeting of the Tat export in Bacillus subtilis.
J Biol Chem: 2003, 278(40);38428-36
[PubMed:12867413]
[WorldCat.org]
[DOI]
(P p)
J D Jongbloed, U Martin, H Antelmann, M Hecker, H Tjalsma, G Venema, S Bron, J M van Dijl, J Müller
TatC is a specificity determinant for protein secretion via the twin-arginine translocation pathway.
J Biol Chem: 2000, 275(52);41350-7
[PubMed:11007775]
[WorldCat.org]
[DOI]
(P p)
S Eder, W Liu, F M Hulett
Mutational analysis of the phoD promoter in Bacillus subtilis: implications for PhoP binding and promoter activation of Pho regulon promoters.
J Bacteriol: 1999, 181(7);2017-25
[PubMed:10094677]
[WorldCat.org]
[DOI]
(P p)