Difference between revisions of "CcpN"
(→Biological materials) |
(→Biological materials) |
||
| Line 132: | Line 132: | ||
* '''Mutant:''' | * '''Mutant:''' | ||
| − | **DB104 ''ccpN''::cat, available in [[Sabine Brantl]] lab | + | **DB104 ''ccpN''::cat, available in [[Sabine Brantl]]'s lab |
| − | **GP1128 ''ccpN''::cat, available in [[Stülke]] lab | + | **GP1128 ''ccpN''::cat, available in [[Jörg Stülke]]'s lab |
* '''Expression vector:''' | * '''Expression vector:''' | ||
| Line 141: | Line 141: | ||
* '''GFP fusion:''' | * '''GFP fusion:''' | ||
| − | * '''Antibody:''' available in [[Sabine Brantl]] lab | + | * '''Antibody:''' available in [[Sabine Brantl]]'s lab |
=Labs working on this gene/protein= | =Labs working on this gene/protein= | ||
Revision as of 16:08, 30 October 2012
- Description: transcriptional repressor of gluconeogenetic genes and of sr1. repression in the presence of glucose
| Gene name | ccpN |
| Synonyms | yqzB |
| Essential | no |
| Product | transcriptional regulator |
| Function | repressor of genes involved in gluconeogenesis (gapB, pckA) and of sr1 |
| Gene expression levels in SubtiExpress: ccpN | |
| Interactions involving this protein in SubtInteract: CcpN | |
| Metabolic function and regulation of this protein in SubtiPathways: Ammonium/ glutamate, Cys, Met & Sulfate assimilation, Central C-metabolism | |
| MW, pI | 23.4 kDa, 7.22 |
| Gene length, protein length | 636 bp, 212 amino acids |
| Immediate neighbours | yqfL, glyS |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 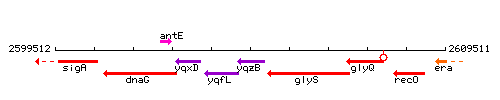
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
carbon core metabolism, transcription factors and their control, regulators of core metabolism
This gene is a member of the following regulons
The CcpN regulon:
The gene
Basic information
- Locus tag: BSU25250
Phenotypes of a mutant
Impaired growth on glucose due to re-routing of carbon from glycolysis to the pentose phosphate pathway PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: transcription repression of the gapB, pckA, and sr1 genes in the presence of glucose PubMed
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure:
- UniProt: O34994
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Sigma factor:
- Regulation: constitutively expressed PubMed
- Additional information:
- the intracellular concentration of CcpN is about 4 myM (according to PubMed).
Biological materials
- Mutant:
- DB104 ccpN::cat, available in Sabine Brantl's lab
- GP1128 ccpN::cat, available in Jörg Stülke's lab
- Expression vector:
- lacZ fusion:
- GFP fusion:
- Antibody: available in Sabine Brantl's lab
Labs working on this gene/protein
Stephane Aymerich, Microbiology and Molecular Genetics, INRA Paris-Grignon, France
Sabine Brantl, Bacterial Genetics, Friedrich-Schiller-University of Jena, Germany homepage
Uwe Sauer, ETH Zürich, Switzerland homepage
Your additional remarks
References
Reviews
Sabine Brantl, Andreas Licht
Characterisation of Bacillus subtilis transcriptional regulators involved in metabolic processes.
Curr Protein Pept Sci: 2010, 11(4);274-91
[PubMed:20408793]
[WorldCat.org]
[DOI]
(I p)
Original Publications