Difference between revisions of "TsaD"
(→Expression and regulation) |
|||
| Line 108: | Line 108: | ||
=Expression and regulation= | =Expression and regulation= | ||
| − | * '''Operon:''' | + | * '''Operon:''' ''[[thiL]]-[[tsaE]]-[[tsaB]]-[[ydiD]]-[[tsaD]]'' {{PubMed|22383849}} |
* '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=gcp_643258_644298_1 tsaD] {{PubMed|22383849}} | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=gcp_643258_644298_1 tsaD] {{PubMed|22383849}} | ||
| Line 139: | Line 139: | ||
=References= | =References= | ||
| − | <pubmed> 23072323 </pubmed> | + | <pubmed> 23072323 22383849</pubmed> |
==Publications on the corresponding'' E. coli'' protein, YgjD== | ==Publications on the corresponding'' E. coli'' protein, YgjD== | ||
<pubmed> 21183954 19376873 20824107 21619589 21285948 </pubmed> | <pubmed> 21183954 19376873 20824107 21619589 21285948 </pubmed> | ||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 11:37, 18 October 2012
- Description: required for threonyl carbamoyl adenosine (t6A) modification of tRNAs that pair with ANN codons in mRNA (together with TsaC), universally conserved protein
| Gene name | tsaD |
| Synonyms | ydiE, gcp |
| Essential | yes PubMed |
| Product | tRNA modification enzyme |
| Function | tRNA modification |
| Gene expression levels in SubtiExpress: tsaD | |
| Interactions involving this protein in SubtInteract: TsaD | |
| MW, pI | 36 kDa, 5.016 |
| Gene length, protein length | 1038 bp, 346 aa |
| Immediate neighbours | ydiD, ydiF |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 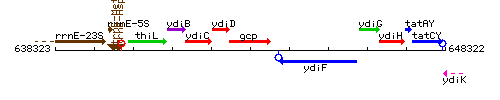
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed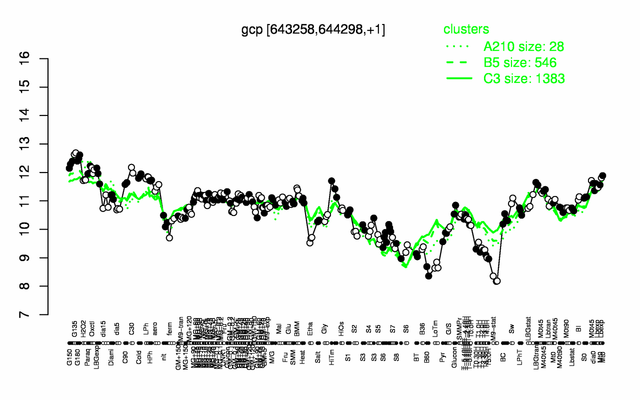
| |
Contents
Categories containing this gene/protein
translation, essential genes, universally conserved proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU05940
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure:
- UniProt: O05518
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Charles T Lauhon
Mechanism of N6-threonylcarbamoyladenonsine (t(6)A) biosynthesis: isolation and characterization of the intermediate threonylcarbamoyl-AMP.
Biochemistry: 2012, 51(44);8950-63
[PubMed:23072323]
[WorldCat.org]
[DOI]
(I p)
Pierre Nicolas, Ulrike Mäder, Etienne Dervyn, Tatiana Rochat, Aurélie Leduc, Nathalie Pigeonneau, Elena Bidnenko, Elodie Marchadier, Mark Hoebeke, Stéphane Aymerich, Dörte Becher, Paola Bisicchia, Eric Botella, Olivier Delumeau, Geoff Doherty, Emma L Denham, Mark J Fogg, Vincent Fromion, Anne Goelzer, Annette Hansen, Elisabeth Härtig, Colin R Harwood, Georg Homuth, Hanne Jarmer, Matthieu Jules, Edda Klipp, Ludovic Le Chat, François Lecointe, Peter Lewis, Wolfram Liebermeister, Anika March, Ruben A T Mars, Priyanka Nannapaneni, David Noone, Susanne Pohl, Bernd Rinn, Frank Rügheimer, Praveen K Sappa, Franck Samson, Marc Schaffer, Benno Schwikowski, Leif Steil, Jörg Stülke, Thomas Wiegert, Kevin M Devine, Anthony J Wilkinson, Jan Maarten van Dijl, Michael Hecker, Uwe Völker, Philippe Bessières, Philippe Noirot
Condition-dependent transcriptome reveals high-level regulatory architecture in Bacillus subtilis.
Science: 2012, 335(6072);1103-6
[PubMed:22383849]
[WorldCat.org]
[DOI]
(I p)
Publications on the corresponding E. coli protein, YgjD
Tobias Bergmiller, Rafael Peña-Miller, Alexander Boehm, Martin Ackermann
Single-cell time-lapse analysis of depletion of the universally conserved essential protein YgjD.
BMC Microbiol: 2011, 11;118
[PubMed:21619589]
[WorldCat.org]
[DOI]
(I e)
Basma El Yacoubi, Isabelle Hatin, Christopher Deutsch, Tamer Kahveci, Jean-Pierre Rousset, Dirk Iwata-Reuyl, Alexey G Murzin, Valérie de Crécy-Lagard
A role for the universal Kae1/Qri7/YgjD (COG0533) family in tRNA modification.
EMBO J: 2011, 30(5);882-93
[PubMed:21285948]
[WorldCat.org]
[DOI]
(I p)
Madhusudhan Srinivasan, Preeti Mehta, Yao Yu, Evelyn Prugar, Eugene V Koonin, A Wali Karzai, Rolf Sternglanz
The highly conserved KEOPS/EKC complex is essential for a universal tRNA modification, t6A.
EMBO J: 2011, 30(5);873-81
[PubMed:21183954]
[WorldCat.org]
[DOI]
(I p)
Chen Katz, Ifat Cohen-Or, Uri Gophna, Eliora Z Ron
The ubiquitous conserved glycopeptidase Gcp prevents accumulation of toxic glycated proteins.
mBio: 2010, 1(3);
[PubMed:20824107]
[WorldCat.org]
[DOI]
(I e)
Jennifer I Handford, Bérengère Ize, Grant Buchanan, Gareth P Butland, Jack Greenblatt, Andrew Emili, Tracy Palmer
Conserved network of proteins essential for bacterial viability.
J Bacteriol: 2009, 191(15);4732-49
[PubMed:19376873]
[WorldCat.org]
[DOI]
(I p)