Difference between revisions of "HtpX"
| Line 105: | Line 105: | ||
=Expression and regulation= | =Expression and regulation= | ||
| − | * '''Operon:''' | + | * '''Operon:''' ''[[ykrL]]'' {{PubMed|22383849}} |
* '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=htpX_1414997_1415893_1 ykrL] {{PubMed|22383849}} | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=htpX_1414997_1415893_1 ykrL] {{PubMed|22383849}} | ||
| Line 112: | Line 112: | ||
* '''Regulation:''' | * '''Regulation:''' | ||
| − | ** induced by membrane protein overproduction ([[YkrK]]) {{PubMed|22447908}} | + | ** induced by membrane protein overproduction ([[YkrK]]) {{PubMed|22624725,22447908}} |
** induced by dissipation of membrane potential (ΔΨ) by addition of a sublethal concentration of valinomycin {{PubMed|22447908}} | ** induced by dissipation of membrane potential (ΔΨ) by addition of a sublethal concentration of valinomycin {{PubMed|22447908}} | ||
** expression is increased due to mutations in ''[[resE]]'', ''[[resB]]'', ''[[sdhC]]'', and'' [[sdhA]]'' {{PubMed|22447908}} | ** expression is increased due to mutations in ''[[resE]]'', ''[[resB]]'', ''[[sdhC]]'', and'' [[sdhA]]'' {{PubMed|22447908}} | ||
| Line 142: | Line 142: | ||
=References= | =References= | ||
| − | <pubmed> 22447908 </pubmed> | + | <pubmed> 22447908 22624725 22383849</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 14:56, 4 June 2012
- Description: similar to E.coli HtpX heat shock protease, stress-responsive membrane protease
| Gene name | ykrL |
| Synonyms | htpX |
| Essential | no |
| Product | membrane protease |
| Function | quality control of membrane proteins |
| MW, pI | 32 kDa, 10.157 |
| Gene length, protein length | 894 bp, 298 aa |
| Immediate neighbours | ykrK, ktrD |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed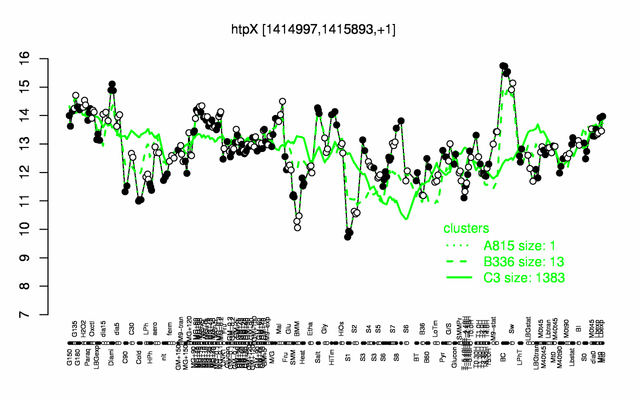
| |
Contents
Categories containing this gene/protein
membrane proteins, proteolysis
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU13490
Phenotypes of a mutant
- increased sensitivity to membrane protein overproduction PubMed
- increased sensitivity to dissipation of the membrane potential by the addition of valinomycin PubMed
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: peptidase M48B family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: cell membrane (according to Swiss-Prot)
Database entries
- Structure:
- UniProt: O31657
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Sigma factor:
- Regulation:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References