Difference between revisions of "FtsZ"
| Line 116: | Line 116: | ||
* '''Operon:''' ''[[ftsA]]-[[ftsZ]]'' [http://www.ncbi.nlm.nih.gov/sites/entrez/1569582 PubMed] | * '''Operon:''' ''[[ftsA]]-[[ftsZ]]'' [http://www.ncbi.nlm.nih.gov/sites/entrez/1569582 PubMed] | ||
| − | * '''[ | + | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=ftsZ_1597832_1598980_1 ftsZ] {{PubMed|22383849}} |
| + | |||
| + | * '''Sigma factor:''' [[SigA]] [http://www.ncbi.nlm.nih.gov/sites/entrez/1569582 PubMed], [[SigH]] [http://www.ncbi.nlm.nih.gov/sites/entrez/1569582 PubMed] | ||
* '''Regulation:''' | * '''Regulation:''' | ||
| Line 123: | Line 125: | ||
* '''Regulatory mechanism:''' | * '''Regulatory mechanism:''' | ||
| − | * '''Additional information:''' | + | * '''Additional information:''' |
=Biological materials = | =Biological materials = | ||
Revision as of 08:30, 13 April 2012
- Description: cell-division initiation protein (septum formation)
| Gene name | ftsZ |
| Synonyms | ts-1 |
| Essential | yes PubMed |
| Product | cell-division initiation protein (septum formation) |
| Function | formation of Z-ring |
| Interactions involving this protein in SubtInteract: FtsZ | |
| MW, pI | 40 kDa, 4.814 |
| Gene length, protein length | 1146 bp, 382 aa |
| Immediate neighbours | ftsA, bpr |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 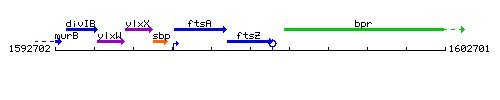
This image was kindly provided by SubtiList
| |
Contents
Categories containing this gene/protein
cell division, essential genes, membrane proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU15290
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: ftsZ family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- UniProt: P17865
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody: available in the Jeff Errington lab
Labs working on this gene/protein
- Imrich Barak, Slovak Academy of Science, Bratislava, Slovakia homepage
- Leendert Hamoen, CBCB, Newcastle University, UK
Your additional remarks
References
Reviews
Clare L Kirkpatrick, Patrick H Viollier
New(s) to the (Z-)ring.
Curr Opin Microbiol: 2011, 14(6);691-7
[PubMed:21981908]
[WorldCat.org]
[DOI]
(I p)
Matthew T Cabeen, Christine Jacobs-Wagner
The bacterial cytoskeleton.
Annu Rev Genet: 2010, 44;365-92
[PubMed:21047262]
[WorldCat.org]
[DOI]
(I p)
Marc Bramkamp, Suey van Baarle
Division site selection in rod-shaped bacteria.
Curr Opin Microbiol: 2009, 12(6);683-8
[PubMed:19884039]
[WorldCat.org]
[DOI]
(I p)
David W Adams, Jeff Errington
Bacterial cell division: assembly, maintenance and disassembly of the Z ring.
Nat Rev Microbiol: 2009, 7(9);642-53
[PubMed:19680248]
[WorldCat.org]
[DOI]
(I p)
Peter L Graumann
Cytoskeletal elements in bacteria.
Annu Rev Microbiol: 2007, 61;589-618
[PubMed:17506674]
[WorldCat.org]
[DOI]
(P p)
Linda A Amos, Fusinita van den Ent, Jan Löwe
Structural/functional homology between the bacterial and eukaryotic cytoskeletons.
Curr Opin Cell Biol: 2004, 16(1);24-31
[PubMed:15037301]
[WorldCat.org]
[DOI]
(P p)
Additional reviews: PubMed
FtsZ as antibacterial drug target
Other original Publications
Additional publications: PubMed