Difference between revisions of "AcoL"
m (Reverted edits by 134.76.70.252 (talk) to last revision by Jstuelk) |
|||
| Line 104: | Line 104: | ||
* '''Operon:''' ''[[acoA]]-[[acoB]]-[[acoC]]-[[acoL]]'' [http://www.ncbi.nlm.nih.gov/sites/entrez/11274109 PubMed] | * '''Operon:''' ''[[acoA]]-[[acoB]]-[[acoC]]-[[acoL]]'' [http://www.ncbi.nlm.nih.gov/sites/entrez/11274109 PubMed] | ||
| − | * '''[ | + | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=acoL_882266_883642_1 acoL] {{PubMed|22383849}} |
| + | |||
| + | * '''Sigma factor:''' [[SigL]] [http://www.ncbi.nlm.nih.gov/sites/entrez/11274109 PubMed] | ||
* '''Regulation:''' | * '''Regulation:''' | ||
Revision as of 16:16, 12 April 2012
- Description: acetoin dehydrogenase E3 component (dihydrolipoamide dehydrogenase)
| Gene name | acoL |
| Synonyms | yfjH |
| Essential | no |
| Product | acetoin dehydrogenase E3 component (dihydrolipoamide dehydrogenase) |
| Function | acetoin utilization |
| Interactions involving this protein in SubtInteract: AcoL | |
| Metabolic function and regulation of this protein in SubtiPathways: Central C-metabolism | |
| MW, pI | 48 kDa, 5.273 |
| Gene length, protein length | 1374 bp, 458 aa |
| Immediate neighbours | acoC, acoR |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 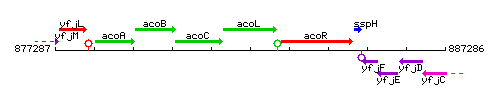
This image was kindly provided by SubtiList
| |
Contents
Categories containing this gene/protein
utilization of specific carbon sources
This gene is a member of the following regulons
AcoR regulon, CcpA regulon, SigL regulon
The gene
Basic information
- Locus tag: BSU08090
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Protein N(6)-(dihydrolipoyl)lysine + NAD+ = protein N(6)-(lipoyl)lysine + NADH (according to Swiss-Prot)
- Protein family: class-I pyridine nucleotide-disulfide oxidoreductase family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- Structure:
- UniProt: O34324
- KEGG entry: [3]
- E.C. number: 1.8.1.4
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Michel Debarbouille, Pasteur Institute, Paris, France Homepage
Your additional remarks
References
Lehnik-Habrink M, Schaffer M, Mäder U, Diethmaier C, Herzberg C, Stülke J RNA processing in Bacillus subtilis: identification of targets of the essential RNase Y. Mol Microbiol. 2011 81(6): 1459-1473. PubMed:21815947