Difference between revisions of "Spx"
| Line 29: | Line 29: | ||
__TOC__ | __TOC__ | ||
| − | + | <br/><br/> | |
Revision as of 22:52, 13 January 2009
- Description: Transcriptional regulator Spx, involved in regulation of many genes.
| Gene name | spx |
| Synonyms | yjbD |
| Essential | no |
| Product | transcriptional regulator Spx |
| Function | negative and positive regulator of many genes |
| MW, pI | 15,5 kDa, 7.80 |
| Gene length, protein length | 393 bp, 131 amino acids |
| Immediate neighbours | yjbC, yjbE |
| Gene sequence (+200bp) | Protein sequence |
Genetic context 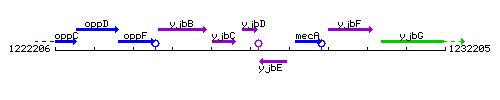
| |
Contents
The gene
Basic information
- Coordinates:
From To Direction Type 1227010 1227402 + CDS 1227427 1227442 + terminator
Phenotypes of a mutant
Loss of up-regulation of the methionine sulfoxide reductase (mrsA-mrsB) operon in response to thiol specific oxidative stress, also loss of trxA and trxB upregulation in response to thiol specific oxidative stress.
Database entries
- DBTBS entry: [1]
- SubtiList entry:
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Transcriptional regulator of many genes in response to thiol specific oxidative stress. In addition, Spx inhibits transcription by binding to the C-terminal domain of the alpha subunit of RNAP (RpoA), disrupting complex formation between RNAP and certain transcriptional activator proteins like ResD and ComA. In response to thiol specific oxidative stress, Spx can also activate transcription, making it a general regulator that exerts both positive and negative control over transcription initiation.
- Protein family: Arsenate Reductase (ArsC) family, Spx subfamily
- Paralogous protein(s): MgsR
Extended information on the protein
- Kinetic information:
- Domains: CXXC (10-13): Acts as a disulfide switch for the redox-sensitive transcriptional regulation of genes that function in thiol homeostasis.
- Modification: Cysteine oxidation of the CXXC motif
- Cofactor(s):
- Effectors of protein activity:
- Interactions: Spx-RpoA (C-terminal domain)
- Localization:
Database entries
- Structure:
- Swiss prot entry:
- KEGG entry:
- E.C. number:
Additional information
Expression and regulation
two promoters upstream of spx: SigA, SigW PubMed
- Regulatory mechanism: transcription repression
- Additional information:Post-translational control by ClpX-ClpP: Spx naturally contains a C-terminal sequence that resembles the SsrA tag and targets the protein for degradation. PubMed
Proteolysis is enhanced by YjbH. PubMed
Biological materials
Labs working on this gene/protein
Peter Zuber, Oregon Health and Science University, USA Homepage
Your additional remarks
References
- Choi, S. Y., D. Reyes, M. Leelakriangsak, and P. Zuber. 2006. The global regulator Spx functions in the control of organosulfur metabolism in Bacillus subtilis. J. Bacteriol. 188:5741-5751. PubMed
- Eiamphungporn, W., and J. D. Helmann. 2008. The Bacillus subtilis sigma(M) regulon and its contribution to cell envelope stress responses. Mol. Microbiol. 67:830-848. PubMed
- Garg et al. 2008. The YjbH protein of Bacillus subtilis enhances ClpXP-catalyzed proteolysis of Spx. J. Bacteriol. in press. PubMed
- Larsson, J. T., A. Rogstam, and C. von Wachenfeldt. 2007. YjbH is a novel negative effector of the disulphide stress regulator, Spx, in Bacillus subtilis. Mol. Microbiol. 66:669-684. PubMed
- Leelakriangsak, M., K. Kobayashi, and P. Zuber. 2007. Dual negative control of spx transcription initiation from the P3 promoter by repressors PerR and YodB in Bacillus subtilis. J. Bacteriol. 189:1736-1744. PubMed
- Nakano, M. M., F. Hajarizadeh, Y. Zhu, and P. Zuber. 2001. Loss-of-function mutations in yjbD result in ClpX- and ClpP-independent competence development of Bacillus subtilis. Mol. Microbiol. 42:383-394.PubMed
- Nakano, S., K. N. Erwin, M. Ralle, and P. Zuber. 2005. Redox-sensitive transcriptional control by a thiol/disulphide switch in the global regulator, Spx. Mol. Microbiol. 55:498-510. PubMed
- Nakano, S., E. Küster-Schöck, A. D. Grossman, and P. Zuber. 2003. Spx dependent global transcriptional control is induced by thiol-specific oxidative stress in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 100:13603-13608. PubMed
- Nakano, S., M. M. Nakano, Y. Zhang, M. Leelakriangsak, and P. Zuber. 2003. A regulatory protein that interferes with activator-stimulated transcription in bacteria. Proc. Natl. Acad. Sci. USA 100:4233-4238. PubMed
- Nakano, S., G. Zheng, M. M. Nakano, and P. Zuber. 2002. Multiple pathways of Spx (YjbD) proteolysis in Bacillus subtilis. J. Bacteriol. 184:3664-3670. PubMed
- Newberry, K. J., S. Nakano, P. Zuber, and R. G. Brennan. 2005. Crystal structure of the Bacillus subtilis anti-alpha, global transcriptional regulator, Spx, in complex with the alpha C-terminal domain of RNA polymerase. Proc. Natl. Acad. Sci. USA 102:15839-15844. PubMed
- Petersohn, A., J. Bernhardt, U. Gerth, D. Hoper, T. Koburger, U. Volker, and M. Hecker. 1999. Identification of sigma(B)-dependent genes in Bacillus subtilis using a promoter consensus-directed search and oligonucleotide hybridization. J. Bacteriol. 181:5718-5724. PubMed
- Reyes, D. Y. and P. Zuber. 2008. Activation of transcription initiation by Spx: formation of a transcription complex and identification of a cis-acting element required for transcriptional activation. Mol. Microbiol. 69:765-779. PubMed
- Thackray, P. D., and A. Moir. 2003. SigM, an extracytoplasmic function sigma factor of Bacillus subtilis, is activated in response to cell wall antibiotics, ethanol, heat, acid, and superoxide stress. J. Bacteriol. 185:3491-3498. PubMed
- Zhang, Y., S. Nakano, S. Y. Choi, and P. Zuber. 2006. Mutational analysis of the Bacillus subtilis RNA polymerase alpha C-terminal domain supports the interference model of Spx-dependent repression. J. Bacteriol. 188:4300-4311. PubMed
- Zhang, Y., and P. Zuber. 2007. Requirement of the zinc-binding domain of ClpX for Spx proteolysis in Bacillus subtilis and effects of disulfide stress on ClpXP activity. J. Bacteriol. 189:7669-7680. PubMed
- Zuber, P. 2004. Spx-RNA polymerase interaction and global transcriptional control during oxidative stress. J. Bacteriol. 186:1911-1918. PubMed