Difference between revisions of "BdbD"
| Line 88: | Line 88: | ||
* '''[[SubtInteract|Interactions]]:''' | * '''[[SubtInteract|Interactions]]:''' | ||
| − | * '''Localization:''' membrane, faced to the outer side of the membrane [http://www.ncbi.nlm.nih.gov/sites/entrez/19535335 PubMed] | + | * '''[[Localization]]:''' membrane, faced to the outer side of the membrane [http://www.ncbi.nlm.nih.gov/sites/entrez/19535335 PubMed] |
=== Database entries === | === Database entries === | ||
Revision as of 09:55, 16 July 2011
- Description: thiol-disulfide oxidoreductase, required for the formation of thiol disulfide bonds in ComGC
| Gene name | bdbD |
| Synonyms | yvgV |
| Essential | no |
| Product | thiol-disulfide oxidoreductase |
| Function | genetic transformation |
| Metabolic function and regulation of this protein in SubtiPathways: Protein secretion | |
| MW, pI | 24 kDa, 5.089 |
| Gene length, protein length | 666 bp, 222 aa |
| Immediate neighbours | bdbC, cadA |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 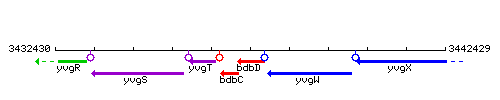
This image was kindly provided by SubtiList
| |
Contents
Categories containing this gene/protein
genetic competence, chaperones/ protein folding, sporulation proteins, membrane proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU33480
Phenotypes of a mutant
loss of transformability PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Protein family: DsbA subfamily (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s): Ca (2+) PubMed
- Effectors of protein activity:
- Localization: membrane, faced to the outer side of the membrane PubMed
Database entries
- UniProt: O32218
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References