Difference between revisions of "YrhJ"
(→Basic information/ Evolution) |
|||
| Line 6: | Line 6: | ||
|''yrhJ'' | |''yrhJ'' | ||
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Synonyms''' || '' '' | + | |style="background:#ABCDEF;" align="center"| '''Synonyms''' || ''cypE '' |
|- | |- | ||
|style="background:#ABCDEF;" align="center"| '''Essential''' || no | |style="background:#ABCDEF;" align="center"| '''Essential''' || no | ||
Revision as of 13:11, 11 August 2010
- Description: similar to cytochrome P450 / NADPH-cytochrome P450 reductase
| Gene name | yrhJ |
| Synonyms | cypE |
| Essential | no |
| Product | NADPH-cytochrome P450 reductase |
| Function | fatty acid metabolism |
| MW, pI | 118 kDa, 6.017 |
| Gene length, protein length | 3162 bp, 1054 aa |
| Immediate neighbours | yrhK, fatR |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 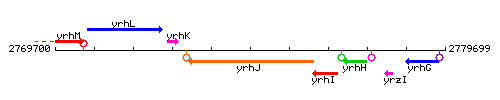
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU27160
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- hydroxylates medium-chain fatty acids in subterminal positions PubMed
- Protein family:
- Paralogous protein(s): YetO
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization: cell membrane (according to Swiss-Prot)
Database entries
- Structure:
- UniProt: O08336
- KEGG entry: [3]
- E.C. number: 1.6.2.4
Additional information
Expression and regulation
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References