Difference between revisions of "AcoL"
| Line 72: | Line 72: | ||
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| − | * '''Interactions:''' | + | * '''Interactions:''' ([[AcoA]]-[[AcoB]])-[[AcoC]]-[[AcoL]] |
* '''Localization:''' cytoplasm (according to Swiss-Prot) | * '''Localization:''' cytoplasm (according to Swiss-Prot) | ||
| Line 100: | Line 100: | ||
* '''Regulatory mechanism:''' | * '''Regulatory mechanism:''' | ||
** [[CcpA]]: transcription repression {{PubMed|10666464}} | ** [[CcpA]]: transcription repression {{PubMed|10666464}} | ||
| − | ** [[AcoR]]: transcription activation (interaction with [[SigL]]-containing RNA polymerase) [http://www.ncbi.nlm.nih.gov/sites/entrez/11274109 PubMed] | + | ** [[AcoR]]: transcription activation (interaction with [[SigL]]-containing [[RNA polymerase]]) [http://www.ncbi.nlm.nih.gov/sites/entrez/11274109 PubMed] |
** [[Fnr]]: indirect positive regulation via [[NarG]]-[[NarH]]-[[NarJ]]-[[NarI]] {{PubMed|16428414}} | ** [[Fnr]]: indirect positive regulation via [[NarG]]-[[NarH]]-[[NarJ]]-[[NarI]] {{PubMed|16428414}} | ||
Revision as of 18:00, 21 August 2010
- Description: acetoin dehydrogenase E3 component (dihydrolipoamide dehydrogenase)
| Gene name | acoL |
| Synonyms | yfjH |
| Essential | no |
| Product | acetoin dehydrogenase E3 component (dihydrolipoamide dehydrogenase) |
| Function | acetoin utilization |
| Metabolic function and regulation of this protein in SubtiPathways: Central C-metabolism | |
| MW, pI | 48 kDa, 5.273 |
| Gene length, protein length | 1374 bp, 458 aa |
| Immediate neighbours | acoC, acoR |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 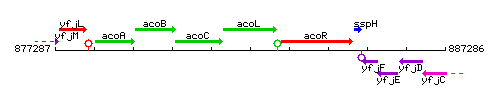
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU08090
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Protein N(6)-(dihydrolipoyl)lysine + NAD+ = protein N(6)-(lipoyl)lysine + NADH (according to Swiss-Prot)
- Protein family: class-I pyridine nucleotide-disulfide oxidoreductase family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- Structure:
- UniProt: O34324
- KEGG entry: [3]
- E.C. number: 1.8.1.4
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:the mRNA is very stable (half-life > 15 min) PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Michel Debarbouille, Pasteur Institute, Paris, France Homepage
Your additional remarks
References
Heike Reents, Richard Münch, Thorben Dammeyer, Dieter Jahn, Elisabeth Härtig
The Fnr regulon of Bacillus subtilis.
J Bacteriol: 2006, 188(3);1103-12
[PubMed:16428414]
[WorldCat.org]
[DOI]
(P p)
G Hambraeus, C von Wachenfeldt, L Hederstedt
Genome-wide survey of mRNA half-lives in Bacillus subtilis identifies extremely stable mRNAs.
Mol Genet Genomics: 2003, 269(5);706-14
[PubMed:12884008]
[WorldCat.org]
[DOI]
(P p)
N O Ali, J Bignon, G Rapoport, M Debarbouille
Regulation of the acetoin catabolic pathway is controlled by sigma L in Bacillus subtilis.
J Bacteriol: 2001, 183(8);2497-504
[PubMed:11274109]
[WorldCat.org]
[DOI]
(P p)
Y Miwa, A Nakata, A Ogiwara, M Yamamoto, Y Fujita
Evaluation and characterization of catabolite-responsive elements (cre) of Bacillus subtilis.
Nucleic Acids Res: 2000, 28(5);1206-10
[PubMed:10666464]
[WorldCat.org]
[DOI]
(I p)
M Huang, F B Oppermann-Sanio, A Steinbüchel
Biochemical and molecular characterization of the Bacillus subtilis acetoin catabolic pathway.
J Bacteriol: 1999, 181(12);3837-41
[PubMed:10368162]
[WorldCat.org]
[DOI]
(P p)