Difference between revisions of "OdhB"
(→Database entries) |
|||
| Line 77: | Line 77: | ||
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| − | * '''Interactions:''' [[OdhA]]-[[OdhB]]-[[PdhD]] | + | * '''Interactions:''' |
| + | ** [[OdhA]]-[[OdhB]]-[[PdhD]] {{PubMed|20933603}} | ||
| + | ** [[OdhB]]-[[Icd]] {{PubMed|20933603}} | ||
* '''Localization:''' cell membrane (according to Swiss-Prot), membrane associated [http://www.ncbi.nlm.nih.gov/pubmed/18763711 PubMed] | * '''Localization:''' cell membrane (according to Swiss-Prot), membrane associated [http://www.ncbi.nlm.nih.gov/pubmed/18763711 PubMed] | ||
| Line 128: | Line 130: | ||
=References= | =References= | ||
| − | <pubmed>2500417,,1508153,12850135 18763711 12682299,11976317 </pubmed> | + | <pubmed>2500417,,1508153,12850135 18763711 12682299,11976317 20933603</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 08:06, 13 October 2010
- Description: 2-oxoglutarate dehydrogenase complex (dihydrolipoamide transsuccinylase, E2 subunit)
| Gene name | odhB |
| Synonyms | citM |
| Essential | yes PubMed |
| Product | 2-oxoglutarate dehydrogenase complex
(dihydrolipoamide transsuccinylase, E2 subunit) |
| Function | TCA cycle |
| Metabolic function and regulation of this protein in SubtiPathways: Central C-metabolism | |
| MW, pI | 45 kDa, 4.859 |
| Gene length, protein length | 1251 bp, 417 aa |
| Immediate neighbours | yocS, odhA |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 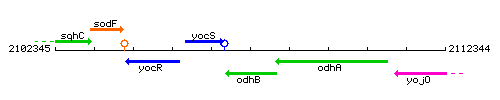
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU19360
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Succinyl-CoA + enzyme N(6)-(dihydrolipoyl)lysine = CoA + enzyme N(6)-(S-succinyldihydrolipoyl)lysine (according to Swiss-Prot)
- Protein family: sodium:bile acid symporter family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: cell membrane (according to Swiss-Prot), membrane associated PubMed
Database entries
- UniProt: P16263
- KEGG entry: [3]
- E.C. number: 2.3.1.61
Additional information
Expression and regulation
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Stülke lab
- Antibody:
Labs working on this gene/protein
Your additional remarks
References