Difference between revisions of "HutH"
(→References) |
(→References) |
||
| Line 125: | Line 125: | ||
=References= | =References= | ||
| − | <pubmed>10746760 ,6143742, 8071225 8682780 </pubmed> | + | <pubmed>10746760 ,6143742, 8071225 8682780 10217782 </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 19:38, 15 February 2010
- Description: histidase
| Gene name | hutH |
| Synonyms | |
| Essential | no |
| Product | histidase |
| Function | histidine utilization |
| Metabolic function and regulation of this protein in SubtiPathways: His | |
| MW, pI | 55 kDa, 5.221 |
| Gene length, protein length | 1524 bp, 508 aa |
| Immediate neighbours | hutP, hutU |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 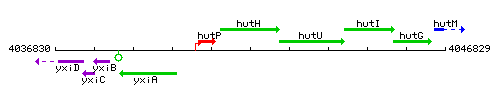
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU39350
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: L-histidine = urocanate + NH3 (according to Swiss-Prot)
- Protein family: PAL/histidase family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- Structure:
- UniProt: P10944
- KEGG entry: [3]
- E.C. number: 4.3.1.3
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant: GP1114 (spc), available in Stülke lab
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Ken-Ichi Yoshida, Izumi Ishio, Eishi Nagakawa, Yoshiyuki Yamamoto, Mami Yamamoto, Yasutaro Fujita
Systematic study of gene expression and transcription organization in the gntZ-ywaA region of the Bacillus subtilis genome.
Microbiology (Reading): 2000, 146 ( Pt 3);573-579
[PubMed:10746760]
[WorldCat.org]
[DOI]
(P p)
J M Zalieckas, L V Wray, S H Fisher
trans-acting factors affecting carbon catabolite repression of the hut operon in Bacillus subtilis.
J Bacteriol: 1999, 181(9);2883-8
[PubMed:10217782]
[WorldCat.org]
[DOI]
(P p)
S H Fisher, K Rohrer, A E Ferson
Role of CodY in regulation of the Bacillus subtilis hut operon.
J Bacteriol: 1996, 178(13);3779-84
[PubMed:8682780]
[WorldCat.org]
[DOI]
(P p)
L V Wray, S H Fisher
Analysis of Bacillus subtilis hut operon expression indicates that histidine-dependent induction is mediated primarily by transcriptional antitermination and that amino acid repression is mediated by two mechanisms: regulation of transcription initiation and inhibition of histidine transport.
J Bacteriol: 1994, 176(17);5466-73
[PubMed:8071225]
[WorldCat.org]
[DOI]
(P p)
S H Fisher, B Magasanik
2-Ketoglutarate and the regulation of aconitase and histidase formation in Bacillus subtilis.
J Bacteriol: 1984, 158(1);379-82
[PubMed:6143742]
[WorldCat.org]
[DOI]
(P p)