Difference between revisions of "Sandbox"
| Line 1: | Line 1: | ||
| − | * '''Description:''' | + | * '''Description:''' Carbon catabolite control protein A, involved in glucose regulation of many genes; represses catabolic genes and activates genes involved in excretion of excess carbon <br/><br/> |
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Gene name''' | |style="background:#ABCDEF;" align="center"|'''Gene name''' | ||
| − | |'' | + | |''ccpA'' |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Synonyms''' || '' '' | + | |style="background:#ABCDEF;" align="center"| '''Synonyms''' || ''graR, alsA, amyR'' |
|- | |- | ||
|style="background:#ABCDEF;" align="center"| '''Essential''' || no | |style="background:#ABCDEF;" align="center"| '''Essential''' || no | ||
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Product''' || | + | |style="background:#ABCDEF;" align="center"| '''Product''' || transcriptional regulator |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"|'''Function''' || | + | |style="background:#ABCDEF;" align="center"|'''Function''' || mediates carbon catabolite repression (CCR) |
|- | |- | ||
| − | |colspan="2" style="background:#FAF8CC;" align="center"| '''Metabolic function and regulation of this protein in [[SubtiPathways|''Subti''Pathways]]: <br/>[http://subtiwiki.uni-goettingen.de/pathways/ | + | |colspan="2" style="background:#FAF8CC;" align="center"| '''Metabolic function and regulation of this protein in [[SubtiPathways|''Subti''Pathways]]: <br/>[http://subtiwiki.uni-goettingen.de/pathways/nucleosides_catabolism.html Nucleoside catabolism], [http://subtiwiki.uni-goettingen.de/pathways/gene_regulation_nucleotides.html Nucleotides (regulation)], [http://subtiwiki.uni-goettingen.de/pathways/ile_val_leu.html Ile, Leu, Val],<br/>[http://subtiwiki.uni-goettingen.de/pathways/histidine.html His], [http://subtiwiki.uni-goettingen.de/pathways/CoA_synthesis.html Coenzyme A], [http://subtiwiki.uni-goettingen.de/pathways/carbon_flow.html Central C-metabolism]''' |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || | + | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || 36,8 kDa, 5.06 |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Gene length, protein length''' || | + | |style="background:#ABCDEF;" align="center"| '''Gene length, protein length''' || 1002 bp, 334 amino acids |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[ | + | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[aroA]]'', ''[[motP]]'' |
|- | |- | ||
| − | |colspan="2" style="background:#FAF8CC;" align="center"|'''Get the DNA and protein [http://srs.ebi.ac.uk/srsbin/cgi-bin/wgetz?-e+[EMBLCDS: | + | |colspan="2" style="background:#FAF8CC;" align="center"|'''Get the DNA and protein [http://srs.ebi.ac.uk/srsbin/cgi-bin/wgetz?-e+[EMBLCDS:CAB14952]+-newId sequences] <br/> (Barbe ''et al.'', 2009)''' |
|- | |- | ||
| − | |colspan="2" | '''Genetic context''' <br/> [[Image: | + | |colspan="2" | '''Genetic context''' <br/> [[Image:ccpA_context.gif]] |
<div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | <div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | ||
|- | |- | ||
| Line 38: | Line 38: | ||
=== Basic information === | === Basic information === | ||
| − | * '''Locus tag:''' | + | * '''Locus tag:''' BSU29740 |
===Phenotypes of a mutant === | ===Phenotypes of a mutant === | ||
| − | + | Loss of carbon catabolite repression. | |
| + | Loss of PTS-dependent sugar transport due to excessive phosphorylation of [[PtsH |HPr]] by [[HprK]]. | ||
| + | The mutant is unable to grow on a minimal medium with glucose and ammonium as the only sources of carbon and nitrogen, respectively. | ||
=== Database entries === | === Database entries === | ||
| − | * '''DBTBS entry:''' [http://dbtbs.hgc.jp/COG/prom/ | + | * '''DBTBS entry:''' [http://dbtbs.hgc.jp/COG/prom/ccpA-motPS.html] |
| − | * '''SubtiList entry:''' [http://genolist.pasteur.fr/SubtiList/genome.cgi?gene_detail+ | + | * '''SubtiList entry:''' [http://genolist.pasteur.fr/SubtiList/genome.cgi?gene_detail+BG10376] |
=== Additional information=== | === Additional information=== | ||
| Line 56: | Line 58: | ||
=== Basic information/ Evolution === | === Basic information/ Evolution === | ||
| − | * '''Catalyzed reaction/ biological activity:''' | + | * '''Catalyzed reaction/ biological activity:''' transcriptional regulator of carbon catabolite repression (CCR) |
| − | * '''Protein family:''' | + | * '''Protein family:''' LacI family |
| − | * '''Paralogous protein(s):''' [[ | + | * '''Paralogous protein(s):''' |
| + | |||
| + | === Genes controlled by CcpA === | ||
| + | |||
| + | * '''Activation by CcpA:''' ''[[pta]]'', ''[[ackA]]'', ''[[ilvB]]-[[ilvH]]-[[ilvC]]-[[leuA]]-[[leuB]]-[[leuC]]-[[leuD]]'' | ||
| + | |||
| + | * '''Repression by CcpA:''' ''[[abbA]], [[amyE]]'', ''[[bglP]]-[[bglH]]'', ''[[bglS]]'', ''[[cccA]]'', ''[[citZ]]-[[icd]]-[[mdh]]'', ''[[levD]]-[[levE]]-[[levF]]-[[levG]]-[[sacC]]'', ''[[licB]]-[[licC]]-[[licA]]-[[licH]]'', ''[[phoP]]-[[phoR]]'', ''[[xylA]]-[[xylB]]'', ''[[xynP]]-[[xynB]]'' | ||
=== Extended information on the protein === | === Extended information on the protein === | ||
| Line 67: | Line 75: | ||
* '''Domains:''' | * '''Domains:''' | ||
| + | ** HTH lacI-type Domain (1 – 58) | ||
| + | ** DNA binding Domain (6 – 25) | ||
* '''Modification:''' | * '''Modification:''' | ||
| − | * '''Cofactor(s):''' | + | * '''Cofactor(s):''' [[PtsH |HPr]]-Ser46-P, Crh-Ser-46-P |
| − | * '''Effectors of protein activity:''' | + | * '''Effectors of protein activity:'''glucose-6-phosphate, fructose-1,6-bisphosphate [http://www.ncbi.nlm.nih.gov/pubmed/17376479?ordinalpos=1&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_DefaultReportPanel.Pubmed_RVDocSum Pubmed] |
| − | * '''Interactions:''' | + | * '''Interactions:''' CcpA-[[PtsH |HPr]] [http://www.ncbi.nlm.nih.gov/pubmed/15369672?ordinalpos=3&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_DefaultReportPanel.Pubmed_RVDocSum PubMed], CcpA-[[Crh]] [http://www.ncbi.nlm.nih.gov/pubmed/16316990?ordinalpos=2&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_DefaultReportPanel.Pubmed_RVDocSum PubMed] |
* '''Localization:''' | * '''Localization:''' | ||
| Line 80: | Line 90: | ||
=== Database entries === | === Database entries === | ||
| − | * '''Structure:''' | + | * '''Structure:''' CcpA-Crh-DNA-complex [http://www.ncbi.nlm.nih.gov/Structure/mmdb/mmdbsrv.cgi?Dopt=s&uid=52326 NCBI], complex with P-Ser-[[PtsH |HPr]] and sulphate ions [http://www.ncbi.nlm.nih.gov/Structure/mmdb/mmdbsrv.cgi?Dopt=s&uid=39857 NCBI] |
| − | * '''Swiss prot entry:''' [http://www.uniprot.org/uniprot/ | + | * '''Swiss prot entry:''' [http://www.uniprot.org/uniprot/P25144 P25144] |
| − | * '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu: | + | * '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu:BSU29740] |
| − | |||
| − | |||
=== Additional information=== | === Additional information=== | ||
| − | |||
=Expression and regulation= | =Expression and regulation= | ||
| − | * '''Operon:''' '' | + | * '''Operon:''' ''[[ccpA]] [[motP]] [[motS]]'' [http://www.ncbi.nlm.nih.gov/sites/entrez/16547058 PubMed] |
| − | * '''Sigma factor:''' | + | * '''Sigma factor:''' |
| − | * '''Regulation:''' | + | * '''Regulation:''' constitutively expressed [http://www.ncbi.nlm.nih.gov/sites/entrez/18757537 PubMed] |
| − | + | * '''Additional information:''' there are about 3.000 molecules of CcpA per cell [http://www.ncbi.nlm.nih.gov/sites/entrez/8000527 PubMed] | |
| − | |||
| − | * '''Additional information:''' | ||
| − | |||
=Biological materials = | =Biological materials = | ||
| − | * '''Mutant:''' | + | * '''Mutant:''' QB5407 (spc), GP302 (erm), GP300 (an in frame deletion of ''[[ccpA]]''), available in [[Stülke]] lab |
| − | * '''Expression vector:''' | + | * '''Expression vector:''' pGP643 (N-terminal Strep-tag, purification from ''B. subtilis'', for [[SPINE]], in [[pGP380]]), available in [[Stülke]] lab |
| − | |||
| − | |||
* '''lacZ fusion:''' | * '''lacZ fusion:''' | ||
| Line 116: | Line 118: | ||
* '''GFP fusion:''' | * '''GFP fusion:''' | ||
| − | * ''' | + | * '''Antibody:''' available in [[Hillen]] and [[Stülke]] labs |
| − | + | =Labs working on this gene/protein= | |
| − | = | + | [[Wolfgang Hillen]], Erlangen University, Germany [http://www.biologie.uni-erlangen.de/mibi/index2.html Homepage] |
| + | |||
| + | [[Richard Brennan]], Houston, Texas, USA [http://www.mdanderson.org/departments/biochem/display.cfm?id=556ef368-6c81-4043-b74f350d41dd06cb&method=displayfull&pn=a8427ebd-d0ff-11d4-80fd00508b603a14 Homepage] | ||
| + | |||
| + | [[Milton H. Saier]], University of California at San Diego, USA [http://biology.ucsd.edu/faculty/saier.html Homepage] | ||
| + | |||
| + | [[Yasutaro Fujita]], University of Fukuyama, Japan | ||
| − | [[ | + | [[Stülke|Jörg Stülke]], University of Göttingen, Germany [http://wwwuser.gwdg.de/~genmibio/stuelke.html Homepage] |
| − | [[ | + | [[Oscar Kuipers]], University of Groningen, The Netherlands |
| − | [http:// | + | [http://molgen.biol.rug.nl/molgen/index.php Homepage] |
=Your additional remarks= | =Your additional remarks= | ||
| Line 131: | Line 139: | ||
=References= | =References= | ||
| − | ''' | + | '''Reviews''' |
| − | <pubmed> | + | |
| − | + | <pubmed> 8598282 , 19202299,14665673,18628769 ,18359269, 18628769 </pubmed> | |
| − | ''' | + | |
| − | <pubmed> | + | '''General and physiological studies''' |
| + | |||
| + | <pubmed>1904524 ,10941796 ,12123463,8000527, 18757537,16547058,14523131 </pubmed> | ||
| + | |||
| + | '''Global analyses (proteome, transcriptome)''' | ||
| + | |||
| + | <pubmed>12850135 ,11251851,10559165, 11160890,17183215 </pubmed> | ||
| + | |||
| + | '''Repression of target genes by CcpA''' | ||
| + | |||
| + | <pubmed>15150224 ,16166551 ,11929549 , 7913927 ,17827291 ,11985717 ,12100558,7592486 </pubmed> | ||
| + | |||
| + | '''Positive regulation of gene expression by CcpA''' | ||
| + | |||
| + | <pubmed>8226682 ,12193635 ,10559153 ,15916605, 9811655 ,10986270 </pubmed> | ||
| + | |||
| + | '''Control of CcpA activity''' | ||
| + | |||
| + | <pubmed>7623661 ,9973552 ,9334231 ,12051938, 9689125 </pubmed> | ||
| + | |||
| + | '''CcpA-DNA interaction''' | ||
| + | |||
| + | <pubmed>8596444 ,10666464 ,15885105,7665492 ,9254709 </pubmed> | ||
| + | |||
| + | '''Functional analysis of CcpA''' | ||
| + | |||
| + | <pubmed>10383986 ,10601226 ,11557150,9252590 ,9988473 </pubmed> | ||
| − | ''' | + | '''Structural analyses''' |
| − | |||
| − | + | <pubmed>15369672 ,16316990 ,17376479 </pubmed> | |
| − | <pubmed> | ||
Revision as of 15:27, 16 July 2009
- Description: Carbon catabolite control protein A, involved in glucose regulation of many genes; represses catabolic genes and activates genes involved in excretion of excess carbon
| Gene name | ccpA |
| Synonyms | graR, alsA, amyR |
| Essential | no |
| Product | transcriptional regulator |
| Function | mediates carbon catabolite repression (CCR) |
| Metabolic function and regulation of this protein in SubtiPathways: Nucleoside catabolism, Nucleotides (regulation), Ile, Leu, Val, His, Coenzyme A, Central C-metabolism | |
| MW, pI | 36,8 kDa, 5.06 |
| Gene length, protein length | 1002 bp, 334 amino acids |
| Immediate neighbours | aroA, motP |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 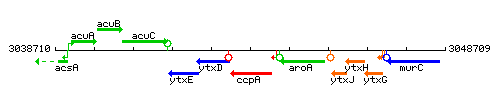
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU29740
Phenotypes of a mutant
Loss of carbon catabolite repression. Loss of PTS-dependent sugar transport due to excessive phosphorylation of HPr by HprK. The mutant is unable to grow on a minimal medium with glucose and ammonium as the only sources of carbon and nitrogen, respectively.
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: transcriptional regulator of carbon catabolite repression (CCR)
- Protein family: LacI family
- Paralogous protein(s):
Genes controlled by CcpA
- Repression by CcpA: abbA, amyE, bglP-bglH, bglS, cccA, citZ-icd-mdh, levD-levE-levF-levG-sacC, licB-licC-licA-licH, phoP-phoR, xylA-xylB, xynP-xynB
Extended information on the protein
- Kinetic information:
- Domains:
- HTH lacI-type Domain (1 – 58)
- DNA binding Domain (6 – 25)
- Modification:
- Cofactor(s): HPr-Ser46-P, Crh-Ser-46-P
- Effectors of protein activity:glucose-6-phosphate, fructose-1,6-bisphosphate Pubmed
- Localization:
Database entries
- Swiss prot entry: P25144
- KEGG entry: [3]
Additional information
Expression and regulation
- Sigma factor:
- Regulation: constitutively expressed PubMed
- Additional information: there are about 3.000 molecules of CcpA per cell PubMed
Biological materials
- Expression vector: pGP643 (N-terminal Strep-tag, purification from B. subtilis, for SPINE, in pGP380), available in Stülke lab
- lacZ fusion:
- GFP fusion:
Labs working on this gene/protein
Wolfgang Hillen, Erlangen University, Germany Homepage
Richard Brennan, Houston, Texas, USA Homepage
Milton H. Saier, University of California at San Diego, USA Homepage
Yasutaro Fujita, University of Fukuyama, Japan
Jörg Stülke, University of Göttingen, Germany Homepage
Oscar Kuipers, University of Groningen, The Netherlands Homepage
Your additional remarks
References
Reviews
General and physiological studies
Global analyses (proteome, transcriptome)
Repression of target genes by CcpA
Positive regulation of gene expression by CcpA
Control of CcpA activity
CcpA-DNA interaction
Functional analysis of CcpA
Structural analyses