Difference between revisions of "Hfq"
(→Biological materials) |
(→Original publications) |
||
| (11 intermediate revisions by 3 users not shown) | |||
| Line 14: | Line 14: | ||
|style="background:#ABCDEF;" align="center"|'''Function''' || unknown | |style="background:#ABCDEF;" align="center"|'''Function''' || unknown | ||
|- | |- | ||
| − | |colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http:// | + | |colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http://subtiwiki.uni-goettingen.de/apps/expression/ ''Subti''Express]''': [http://subtiwiki.uni-goettingen.de/apps/expression/expression.php?search=BSU17340 hfq] |
|- | |- | ||
|style="background:#ABCDEF;" align="center"| '''MW, pI''' || 8 kDa, 8.698 | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || 8 kDa, 8.698 | ||
| Line 22: | Line 22: | ||
|style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[miaA]]'', ''[[ymzC]]'' | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[miaA]]'', ''[[ymzC]]'' | ||
|- | |- | ||
| − | | | + | |style="background:#FAF8CC;" align="center"|'''Sequences'''||[http://bsubcyc.org/BSUB/sequence-aa?type=GENE&object=BSU17340 Protein] [http://bsubcyc.org/BSUB/sequence?type=GENE&object=BSU17340 DNA] [http://bsubcyc.org/BSUB/seq-selector?chromosome=CHROM-1&object=BSU17340 DNA_with_flanks] |
|- | |- | ||
|colspan="2" | '''Genetic context''' <br/> [[Image:ymaH_context.gif]] | |colspan="2" | '''Genetic context''' <br/> [[Image:ymaH_context.gif]] | ||
<div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | <div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | ||
|- | |- | ||
| − | |colspan="2" |'''[http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=ymaH_1867373_1867594_1 Expression at a glance]'''   {{PubMed|22383849}}<br/>[[Image:hfq_expression.png|500px]] | + | |colspan="2" |'''[http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=ymaH_1867373_1867594_1 Expression at a glance]'''   {{PubMed|22383849}}<br/>[[Image:hfq_expression.png|500px|link=http://subtiwiki.uni-goettingen.de/apps/expression/expression.php?search=BSU17340]] |
|- | |- | ||
|} | |} | ||
| Line 41: | Line 41: | ||
= This gene is a member of the following [[regulons]] = | = This gene is a member of the following [[regulons]] = | ||
| − | |||
=The gene= | =The gene= | ||
| Line 52: | Line 51: | ||
=== Database entries === | === Database entries === | ||
| + | * '''BsubCyc:''' [http://bsubcyc.org/BSUB/NEW-IMAGE?type=NIL&object=BSU17340&redirect=T BSU17340] | ||
* '''DBTBS entry:''' no entry | * '''DBTBS entry:''' no entry | ||
| Line 89: | Line 89: | ||
=== Database entries === | === Database entries === | ||
| + | * '''BsubCyc:''' [http://bsubcyc.org/BSUB/NEW-IMAGE?type=NIL&object=BSU17340&redirect=T BSU17340] | ||
* '''Structure:''' [http://www.pdb.org/pdb/explore/explore.do?structureId=3HSB 3HSB] (complex with an RNA aptamer) {{PubMed|22053080}} | * '''Structure:''' [http://www.pdb.org/pdb/explore/explore.do?structureId=3HSB 3HSB] (complex with an RNA aptamer) {{PubMed|22053080}} | ||
| Line 102: | Line 103: | ||
=Expression and regulation= | =Expression and regulation= | ||
| − | * '''Operon:''' | + | * '''Operon:''' ''[[hfq]]'' {{PubMed|23457461}} |
* '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=ymaH_1867373_1867594_1 hfq] {{PubMed|22383849}} | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=ymaH_1867373_1867594_1 hfq] {{PubMed|22383849}} | ||
| − | * '''Sigma factor:''' | + | * '''[[Sigma factor]]:''' |
| − | * '''Regulation:''' repressed by glucose (7.7-fold) [http://www.ncbi.nlm.nih.gov/pubmed/12850135 PubMed] | + | * '''Regulation:''' |
| + | ** repressed by glucose (7.7-fold) [http://www.ncbi.nlm.nih.gov/pubmed/12850135 PubMed] | ||
| + | ** expression (mRNA levels) is quite constant during growth in minimal medium {{PubMed|23457461}} | ||
| + | ** the Hfq protein amount increases upon transition to stationary phase {{PubMed|23457461}} | ||
* '''Regulatory mechanism:''' | * '''Regulatory mechanism:''' | ||
* '''Additional information:''' | * '''Additional information:''' | ||
| + | ** number of protein molecules per cell (complex medium with amino acids, without glucose): 46 {{PubMed|24696501}} | ||
=Biological materials = | =Biological materials = | ||
| − | * '''Mutant:''' GP22 (cat), available in the [[Stülke]] lab | + | * '''Mutant:''' GP22 (cat), available in the [[Jörg Stülke]]'s lab |
* '''Expression vector:''' | * '''Expression vector:''' | ||
| − | * '''lacZ fusion:''' pGP460 (in [[pAC7]]), available in [[Stülke]] lab | + | * '''lacZ fusion:''' pGP460 (in [[pAC7]]), available in [[Jörg Stülke]]'s lab |
* '''GFP fusion:''' | * '''GFP fusion:''' | ||
| − | * '''two-hybrid system:''' B. pertussis adenylate cyclase-based bacterial two hybrid system ([[BACTH]]), available in [[Stülke]] lab | + | * '''two-hybrid system:''' B. pertussis adenylate cyclase-based bacterial two hybrid system ([[BACTH]]), available in [[Jörg Stülke]]'s lab |
* '''FLAG-tag construct:''' | * '''FLAG-tag construct:''' | ||
| Line 140: | Line 145: | ||
==Original publications== | ==Original publications== | ||
| − | + | <pubmed>12850135, 20445260 23457461 22965117,22053080 24932523 25150227 25915524</pubmed> | |
| − | <pubmed>12850135, 20445260 </pubmed> | ||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Latest revision as of 15:41, 29 April 2015
- Description: RNA chaperone
| Gene name | hfq |
| Synonyms | ymaH |
| Essential | no |
| Product | RNA chaperone |
| Function | unknown |
| Gene expression levels in SubtiExpress: hfq | |
| MW, pI | 8 kDa, 8.698 |
| Gene length, protein length | 219 bp, 73 aa |
| Immediate neighbours | miaA, ymzC |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 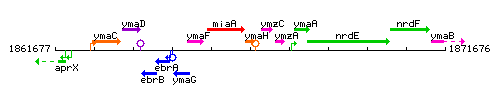
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed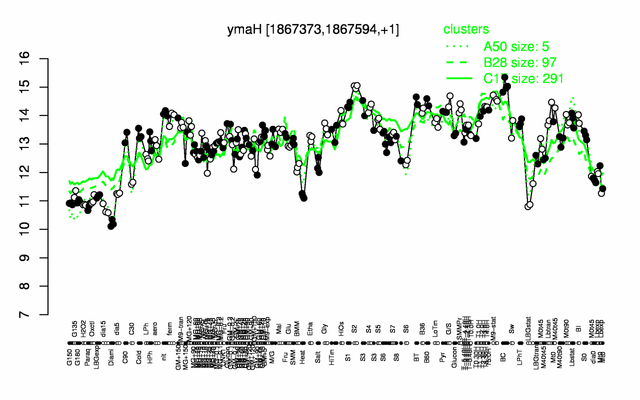
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU17340
Phenotypes of a mutant
Database entries
- BsubCyc: BSU17340
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: hfq family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- BsubCyc: BSU17340
- UniProt: O31796
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
- number of protein molecules per cell (complex medium with amino acids, without glucose): 46 PubMed
Biological materials
- Mutant: GP22 (cat), available in the Jörg Stülke's lab
- Expression vector:
- lacZ fusion: pGP460 (in pAC7), available in Jörg Stülke's lab
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Jörg Stülke's lab
- FLAG-tag construct:
- GP1067 (spc, based on pGP1331), available in Jörg Stülke's lab
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Original publications
Tatiana Rochat, Olivier Delumeau, Nara Figueroa-Bossi, Philippe Noirot, Lionello Bossi, Etienne Dervyn, Philippe Bouloc
Tracking the Elusive Function of Bacillus subtilis Hfq.
PLoS One: 2015, 10(4);e0124977
[PubMed:25915524]
[WorldCat.org]
[DOI]
(I e)
Alexander R Kovach, Kirsten E Hoff, John T Canty, Jillian Orans, Richard G Brennan
Recognition of U-rich RNA by Hfq from the Gram-positive pathogen Listeria monocytogenes.
RNA: 2014, 20(10);1548-59
[PubMed:25150227]
[WorldCat.org]
[DOI]
(I p)
Hermann Hämmerle, Fabian Amman, Branislav Večerek, Jörg Stülke, Ivo Hofacker, Udo Bläsi
Impact of Hfq on the Bacillus subtilis transcriptome.
PLoS One: 2014, 9(6);e98661
[PubMed:24932523]
[WorldCat.org]
[DOI]
(I e)
Michael Dambach, Irnov Irnov, Wade C Winkler
Association of RNAs with Bacillus subtilis Hfq.
PLoS One: 2013, 8(2);e55156
[PubMed:23457461]
[WorldCat.org]
[DOI]
(I p)
Nicola Horstmann, Jillian Orans, Poul Valentin-Hansen, Samuel A Shelburne, Richard G Brennan
Structural mechanism of Staphylococcus aureus Hfq binding to an RNA A-tract.
Nucleic Acids Res: 2012, 40(21);11023-35
[PubMed:22965117]
[WorldCat.org]
[DOI]
(I p)
Tatsuhiko Someya, Seiki Baba, Mai Fujimoto, Gota Kawai, Takashi Kumasaka, Kouji Nakamura
Crystal structure of Hfq from Bacillus subtilis in complex with SELEX-derived RNA aptamer: insight into RNA-binding properties of bacterial Hfq.
Nucleic Acids Res: 2012, 40(4);1856-67
[PubMed:22053080]
[WorldCat.org]
[DOI]
(I p)
Seiki Baba, Tatsuhiko Someya, Gota Kawai, Kouji Nakamura, Takashi Kumasaka
Expression, crystallization and preliminary crystallographic analysis of RNA-binding protein Hfq (YmaH) from Bacillus subtilis in complex with an RNA aptamer.
Acta Crystallogr Sect F Struct Biol Cryst Commun: 2010, 66(Pt 5);563-6
[PubMed:20445260]
[WorldCat.org]
[DOI]
(I p)
Hans-Matti Blencke, Georg Homuth, Holger Ludwig, Ulrike Mäder, Michael Hecker, Jörg Stülke
Transcriptional profiling of gene expression in response to glucose in Bacillus subtilis: regulation of the central metabolic pathways.
Metab Eng: 2003, 5(2);133-49
[PubMed:12850135]
[WorldCat.org]
[DOI]
(P p)