Difference between revisions of "LicT"
(→References) |
|||
| Line 129: | Line 129: | ||
'''Original description''' | '''Original description''' | ||
| − | + | <pubmed>8606172 </pubmed> | |
'''Control of LicT activity''' | '''Control of LicT activity''' | ||
| − | + | <pubmed>10048041, 12169607,8626332, 11580842</pubmed> | |
| − | |||
| − | |||
| − | |||
'''Structural analysis of LicT''' | '''Structural analysis of LicT''' | ||
| − | + | <pubmed>11733988,15699035,11447120,18682383 </pubmed> | |
| − | |||
| − | |||
| − | |||
'''LicT-RNA interaction''' | '''LicT-RNA interaction''' | ||
| − | + | <pubmed>11953318,1279678, 10610766,18682383</pubmed> | |
| − | + | ||
| − | |||
| − | |||
# Author1, Author2 & Author3 (year) Title ''Journal'' '''volume:''' page-page. [http://www.ncbi.nlm.nih.gov/sites/entrez/PMID PubMed] | # Author1, Author2 & Author3 (year) Title ''Journal'' '''volume:''' page-page. [http://www.ncbi.nlm.nih.gov/sites/entrez/PMID PubMed] | ||
Revision as of 20:13, 30 May 2009
- Description: transcriptional antiterminator of the bglPH operon
| Gene name | licT |
| Synonyms | |
| Essential | no |
| Product | transcriptional antiterminator (BglG family) |
| Function | required for substrate-dependent induction of bglPH |
| MW, pI | 32 kDa, 5.944 |
| Gene length, protein length | 831 bp, 277 aa |
| Immediate neighbours | bglS, yxiP |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 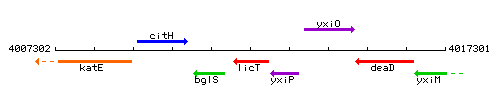
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Coordinates:
Phenotypes of a mutant
no expression of the bglP-bglH operon
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: binding to the mRNAs of bglS and the bglP-bglH operon, causes transcription antitermination (in presence of salicin and absence of glucose)
- Protein family: transcriptional antiterminator bglG family (according to Swiss-Prot) BglG family of antiterminators
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization:
Database entries
- Swiss prot entry: P39805
- KEGG entry: BSU39080
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Stephane Aymerich, Microbiology and Molecular Genetics, INRA Paris-Grignon, France
Josef Deutscher, Microbiology and Molecular Genetics, INRA Paris-Grignon, France
Michael Hecker, Greifswald, Germany Homepage
Your additional remarks
References
Original description
Control of LicT activity
Structural analysis of LicT
LicT-RNA interaction
- Author1, Author2 & Author3 (year) Title Journal volume: page-page. PubMed