Difference between revisions of "MraY"
| Line 93: | Line 93: | ||
* '''Operon:''' [[murE]]-[[mraY]]-[[murD]]-[[spoVE]]-[[murG]]-[[murB]]-[[divIB]]-[[ylxW]]-[[ylxX]]-[[sbp]] | * '''Operon:''' [[murE]]-[[mraY]]-[[murD]]-[[spoVE]]-[[murG]]-[[murB]]-[[divIB]]-[[ylxW]]-[[ylxX]]-[[sbp]] | ||
| − | * '''Sigma factor:''' [[SigE]] | + | * '''[[Sigma factor]]:''' [[SigE]] |
* '''Regulation:''' | * '''Regulation:''' | ||
Revision as of 16:58, 7 April 2009
- Description: phospho-N-acetylmuramoyl-pentapeptide-transferase (meso-2,6-diaminopimelate)
| Gene name | mraY |
| Synonyms | |
| Essential | yes PubMed |
| Product | phospho-N-acetylmuramoyl-pentapeptide-transferase (meso-2,6-diaminopimelate) |
| Function | peptidoglycan precursor biosynthesis |
| MW, pI | 35 kDa, 8.966 |
| Gene length, protein length | 972 bp, 324 aa |
| Immediate neighbours | murE, murD |
| Gene sequence (+200bp) | Protein sequence |
Genetic context 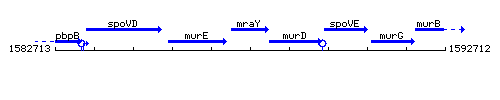
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Coordinates:
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization:
Database entries
- Structure:
- Swiss prot entry:
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
- Al-Dabbagh B, Henry X, El Ghachi M, Auger G, Blanot D, Parquet C, Mengin-Lecreulx D, Bouhss A. (2008) Active site mapping of MraY, a member of the polyprenyl-phosphate N-acetylhexosamine 1-phosphate transferase superfamily, catalyzing the first membrane step of peptidoglycan biosynthesis. Biochemistry. Aug 26;47(34): 8919-28. PubMed
- Author1, Author2 & Author3 (year) Title Journal volume: page-page. PubMed