Difference between revisions of "YkuI"
| Line 1: | Line 1: | ||
| − | * '''Description:''' | + | * '''Description:''' c-di-GMP specific phosphodiesterase <br/><br/> |
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
| Line 10: | Line 10: | ||
|style="background:#ABCDEF;" align="center"| '''Essential''' || no | |style="background:#ABCDEF;" align="center"| '''Essential''' || no | ||
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Product''' || | + | |style="background:#ABCDEF;" align="center"| '''Product''' || c-di-GMP specific phosphodiesterase |
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Function''' || unknown | |style="background:#ABCDEF;" align="center"|'''Function''' || unknown | ||
| Line 52: | Line 52: | ||
=== Basic information/ Evolution === | === Basic information/ Evolution === | ||
| − | * '''Catalyzed reaction/ biological activity:''' | + | * '''Catalyzed reaction/ biological activity:''' degradation of cyclic di-GMP [http://www.ncbi.nlm.nih.gov/sites/entrez/19244251 PubMed] |
* '''Protein family:''' | * '''Protein family:''' | ||
| Line 62: | Line 62: | ||
* '''Kinetic information:''' | * '''Kinetic information:''' | ||
| − | * '''Domains:''' | + | * '''Domains:''' EAL domain (aa 1 ... 245, for c-di-GMP hydrolysis), PAS-like domain (aa 300 ... 400) [http://www.ncbi.nlm.nih.gov/sites/entrez/19244251 PubMed] |
* '''Modification:''' | * '''Modification:''' | ||
| Line 76: | Line 76: | ||
=== Database entries === | === Database entries === | ||
| − | * '''Structure:''' | + | * '''Structure:''' [http://www.rcsb.org/pdb/cgi/explore.cgi?pdbId=2BAS 2BAS], in complex with c-di-GMP [http://www.rcsb.org/pdb/cgi/explore.cgi?pdbId=2W27 2W27] [http://www.ncbi.nlm.nih.gov/sites/entrez/19244251 PubMed] |
* '''Swiss prot entry:''' | * '''Swiss prot entry:''' | ||
| Line 118: | Line 118: | ||
=References= | =References= | ||
| + | # Minasov et al. (2009) Crystal structures of YkuI and its complex with second messenger c-di-GMP suggests catalytic mechanism of phosphodiester bond cleavage by EAL domains ''J. Biol. Chem'' '''volume:''' page-page. [http://www.ncbi.nlm.nih.gov/sites/entrez/19244251 PubMed] | ||
# Author1, Author2 & Author3 (year) Title ''Journal'' '''volume:''' page-page. [http://www.ncbi.nlm.nih.gov/sites/entrez/PMID PubMed] | # Author1, Author2 & Author3 (year) Title ''Journal'' '''volume:''' page-page. [http://www.ncbi.nlm.nih.gov/sites/entrez/PMID PubMed] | ||
Revision as of 11:41, 3 March 2009
- Description: c-di-GMP specific phosphodiesterase
| Gene name | ykuI |
| Synonyms | |
| Essential | no |
| Product | c-di-GMP specific phosphodiesterase |
| Function | unknown |
| MW, pI | 47 kDa, 4.462 |
| Gene length, protein length | 1221 bp, 407 aa |
| Immediate neighbours | ykuH, fsrA |
| Gene sequence (+200bp) | Protein sequence |
Genetic context 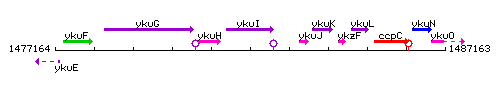
| |
Contents
The gene
Basic information
- Coordinates:
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: degradation of cyclic di-GMP PubMed
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains: EAL domain (aa 1 ... 245, for c-di-GMP hydrolysis), PAS-like domain (aa 300 ... 400) PubMed
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization:
Database entries
- Swiss prot entry:
- KEGG entry:
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody: