Difference between revisions of "KinB"
(→References) |
|||
| Line 153: | Line 153: | ||
=References= | =References= | ||
| − | <pubmed>8576055,16166384,9299348,8497199,10094672,9426145,11069677,12618455,11902725,12618455,7592498, 21097618 19101565 23378509 23599347 </pubmed> | + | <pubmed>8576055,16166384,9299348,8497199,10094672,9426145,11069677,12618455,11902725,12618455,7592498, 21097618 19101565 23378509 23599347 26152584</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 14:05, 13 July 2015
- Description: two-component sensor kinase, phosphorylates Spo0F, part of the phosphorelay, senses changes in respiratory activity
| Gene name | kinB |
| Synonyms | |
| Essential | no |
| Product | two-component sensor kinase |
| Function | initiation of sporulation |
| Gene expression levels in SubtiExpress: kinB | |
| Interactions involving this protein in SubtInteract: KinB | |
| Function and regulation of this protein in SubtiPathways: kinB | |
| MW, pI | 47 kDa, 6.682 |
| Gene length, protein length | 1287 bp, 429 aa |
| Immediate neighbours | patB, kapB |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 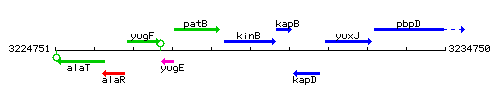
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed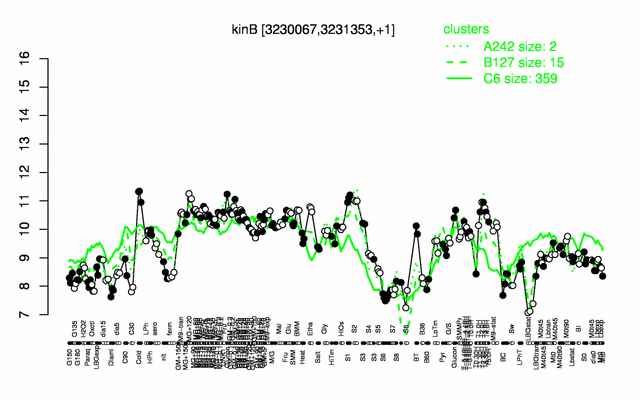
| |
Contents
Categories containing this gene/protein
protein modification, transcription factors and their control, phosphorelay, membrane proteins, phosphoproteins
This gene is a member of the following regulons
CodY regulon, stringent response
The gene
Basic information
- Locus tag: BSU31450
Phenotypes of a mutant
Database entries
- BsubCyc: BSU31450
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains: six transmembrane segments, C-terminal histidine phosphotransferase domain
- Modification: autophosphorylation on a His residue
- Cofactor(s):
- Effectors of protein activity:
- activity is triggered at low respiratory activity, this depends on a functional interaction with the respiration apparatus PubMed
- Localization: cell membrane (integral membrane protein) PubMed
Database entries
- BsubCyc: BSU31450
- UniProt: Q08430
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- repressed during growth in the presence of branched chain amino acids (CodY) PubMed
- induced upon addition of decoyinine (positive stringent response) PubMed
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References