Difference between revisions of "Gmk"
(→References) |
(→References) |
||
| Line 145: | Line 145: | ||
=References= | =References= | ||
| − | <pubmed>22517742 22981860 24722991 24682323</pubmed> | + | <pubmed>22517742 22981860 24722991 24682323 25661490</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 17:28, 13 February 2015
- Description: guanylate kinase (GMP:dATP, dGMP:ATP)
| Gene name | gmk |
| Synonyms | yloD |
| Essential | yes PubMed |
| Product | guanylate kinase (GMP:dATP, dGMP:ATP) |
| Function | GTP biosynthesis |
| Gene expression levels in SubtiExpress: gmk | |
| Metabolic function and regulation of this protein in SubtiPathways: gmk | |
| MW, pI | 23 kDa, 4.616 |
| Gene length, protein length | 612 bp, 204 aa |
| Immediate neighbours | ylzA, yloH |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 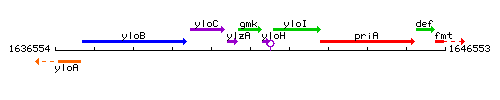
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
biosynthesis/ acquisition of nucleotides, essential genes, phosphoproteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU15680
Phenotypes of a mutant
essential PubMed
Database entries
- BsubCyc: BSU15680
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: ATP + GMP = ADP + GDP (according to Swiss-Prot)
- Protein family: guanylate kinase-like domain (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- phosphorylated on Arg-134 and/or Arg-149PubMed
- Cofactor(s):
- Effectors of protein activity:
- inhibition of enzymatic activity by (p)ppGpp during the ´stringent response´PubMed
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- BsubCyc: BSU15680
- Structure: 1S4Q (from Mycobacterium tuberculosis h37rv, 39% identity, 60% similarity)
- UniProt: O34328
- KEGG entry: [2]
- E.C. number: 2.7.4.8
Additional information
Expression and regulation
- Operon:
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
- number of protein molecules per cell (minimal medium with glucose and ammonium): 254 PubMed
- number of protein molecules per cell (complex medium with amino acids, without glucose): 1208 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, exponential phase): 1529 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, early stationary phase after glucose exhaustion): 895 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, late stationary phase after glucose exhaustion): 1794 PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Kuanqing Liu, Angela R Myers, Tippapha Pisithkul, Kathy R Claas, Kenneth A Satyshur, Daniel Amador-Noguez, James L Keck, Jue D Wang
Molecular mechanism and evolution of guanylate kinase regulation by (p)ppGpp.
Mol Cell: 2015, 57(4);735-749
[PubMed:25661490]
[WorldCat.org]
[DOI]
(I p)
Yuhta Nomura, Atsushi Izumi, Yoshinori Fukunaga, Kensuke Kusumi, Koh Iba, Seiya Watanabe, Yoichi Nakahira, Andreas P M Weber, Akira Nozawa, Yuzuru Tozawa
Diversity in guanosine 3',5'-bisdiphosphate (ppGpp) sensitivity among guanylate kinases of bacteria and plants.
J Biol Chem: 2014, 289(22);15631-41
[PubMed:24722991]
[WorldCat.org]
[DOI]
(I p)
Alycia N Bittner, Allison Kriel, Jue D Wang
Lowering GTP level increases survival of amino acid starvation but slows growth rate for Bacillus subtilis cells lacking (p)ppGpp.
J Bacteriol: 2014, 196(11);2067-76
[PubMed:24682323]
[WorldCat.org]
[DOI]
(I p)
Allison Kriel, Alycia N Bittner, Sok Ho Kim, Kuanqing Liu, Ashley K Tehranchi, Winnie Y Zou, Samantha Rendon, Rui Chen, Benjamin P Tu, Jue D Wang
Direct regulation of GTP homeostasis by (p)ppGpp: a critical component of viability and stress resistance.
Mol Cell: 2012, 48(2);231-41
[PubMed:22981860]
[WorldCat.org]
[DOI]
(I p)
Alexander K W Elsholz, Kürsad Turgay, Stephan Michalik, Bernd Hessling, Katrin Gronau, Dan Oertel, Ulrike Mäder, Jörg Bernhardt, Dörte Becher, Michael Hecker, Ulf Gerth
Global impact of protein arginine phosphorylation on the physiology of Bacillus subtilis.
Proc Natl Acad Sci U S A: 2012, 109(19);7451-6
[PubMed:22517742]
[WorldCat.org]
[DOI]
(I p)