Difference between revisions of "LysS"
| Line 127: | Line 127: | ||
* '''Additional information:''' | * '''Additional information:''' | ||
| + | ** number of protein molecules per cell (minimal medium with glucose and ammonium): 1972 {{PubMed|24696501}} | ||
| + | ** number of protein molecules per cell (complex medium with amino acids, without glucose): 7155 {{PubMed|24696501}} | ||
=Biological materials = | =Biological materials = | ||
Revision as of 09:46, 17 April 2014
- Description: lysyl-tRNA synthetase
| Gene name | lysS |
| Synonyms | |
| Essential | yes PubMed |
| Product | lysyl-tRNA synthetase |
| Function | translation |
| Gene expression levels in SubtiExpress: lysS | |
| Metabolic function and regulation of this protein in SubtiPathways: lysS | |
| MW, pI | 57 kDa, 5.034 |
| Gene length, protein length | 1497 bp, 499 aa |
| Immediate neighbours | yacF, rrnJ-16S |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 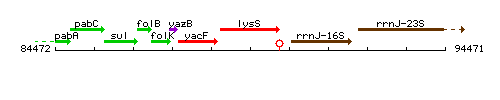
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed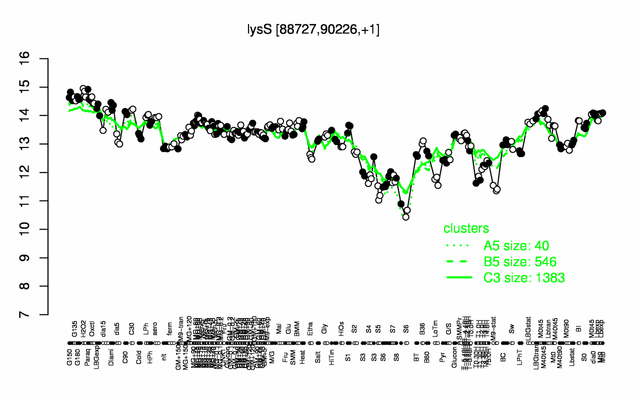
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU00820
Phenotypes of a mutant
essential PubMed
Database entries
- BsubCyc: BSU00820
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: ATP + L-lysine + tRNA(Lys) = AMP + diphosphate + L-lysyl-tRNA(Lys) (according to Swiss-Prot)
- Protein family: class-II aminoacyl-tRNA synthetase family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization:
- cytoplasm (according to Swiss-Prot)
Database entries
- BsubCyc: BSU00820
- Structure: 2E9I (from Geobacillus stearothermophilus, in complex with L-Lysine hydroxamate-AMP) PubMed
- UniProt: P37477
- KEGG entry: [3]
- E.C. number: 6.1.1.6
Additional information
Expression and regulation
lysS PubMed
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Haruko Sakurama, Teisuke Takita, Bunzo Mikami, Takafumi Itoh, Kiyoshi Yasukawa, Kuniyo Inouye
Two crystal structures of lysyl-tRNA synthetase from Bacillus stearothermophilus in complex with lysyladenylate-like compounds: insights into the irreversible formation of the enzyme-bound adenylate of L-lysine hydroxamate.
J Biochem: 2009, 145(5);555-63
[PubMed:19174549]
[WorldCat.org]
[DOI]
(I p)
Antoine de Saizieu, Pierre Vankan, Cassandra Vockler, Adolphus P G M van Loon
The trp RNA-binding attenuation protein (TRAP) regulates the steady-state levels of transcripts of the Bacillus subtilis folate operon.
Microbiology (Reading): 1997, 143 ( Pt 3);979-989
[PubMed:9084182]
[WorldCat.org]
[DOI]
(P p)