Difference between revisions of "AtpA"
| Line 42: | Line 42: | ||
{{SubtiWiki category|[[ATP synthesis]]}}, | {{SubtiWiki category|[[ATP synthesis]]}}, | ||
{{SubtiWiki category|[[membrane proteins]]}}, | {{SubtiWiki category|[[membrane proteins]]}}, | ||
| − | {{SubtiWiki category|[[phosphoproteins]]}} | + | {{SubtiWiki category|[[phosphoproteins]]}}, |
| + | [[most abundant proteins]] | ||
= This gene is a member of the following [[regulons]] = | = This gene is a member of the following [[regulons]] = | ||
| Line 62: | Line 63: | ||
=== Additional information=== | === Additional information=== | ||
| − | |||
| − | |||
| − | |||
=The protein= | =The protein= | ||
| Line 80: | Line 78: | ||
* '''Kinetic information:''' | * '''Kinetic information:''' | ||
| − | * '''Domains:''' | + | * '''[[Domains]]:''' |
* '''Modification:''' phosphorylated on ser/ thr/ tyr [http://www.ncbi.nlm.nih.gov/pubmed/16493705 PubMed] | * '''Modification:''' phosphorylated on ser/ thr/ tyr [http://www.ncbi.nlm.nih.gov/pubmed/16493705 PubMed] | ||
| − | * ''' | + | * '''[[Cofactors]]:''' |
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| Line 121: | Line 119: | ||
* '''Additional information:''' | * '''Additional information:''' | ||
| + | ** belongs to the 100 [[most abundant proteins]] {{PubMed|15378759}} | ||
=Biological materials = | =Biological materials = | ||
| Line 144: | Line 143: | ||
<pubmed> 23356252 23341301, 23267178 22822068 21524994 19489730 17208001 16730323 </pubmed> | <pubmed> 23356252 23341301, 23267178 22822068 21524994 19489730 17208001 16730323 </pubmed> | ||
== Original publications == | == Original publications == | ||
| − | <pubmed>7961438,,16493705 18763711, </pubmed> | + | <pubmed>7961438,15378759,16493705 18763711, </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 16:16, 5 March 2014
- Description: ATP synthase, part of the F1 complex (subunit alpha)
| Gene name | atpA |
| Synonyms | |
| Essential | no |
| Product | ATP synthase (subunit alpha)) |
| Function | ATP synthesis |
| Gene expression levels in SubtiExpress: atpA | |
| Interactions involving this protein in SubtInteract: AtpA | |
| MW, pI | 54 kDa, 5.04 |
| Gene length, protein length | 1506 bp, 502 aa |
| Immediate neighbours | atpG, atpH |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 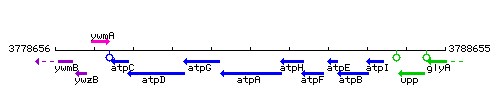
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
ATP synthesis, membrane proteins, phosphoproteins, most abundant proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU36830
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: ATP + H2O + H+(In) = ADP + phosphate + H+(Out) (according to Swiss-Prot) see a video
- Protein family: ATPase alpha/beta chains family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Modification: phosphorylated on ser/ thr/ tyr PubMed
- Effectors of protein activity:
- Localization:
- membrane PubMed
- peripheral via theF0 complex
Database entries
- Structure: see here an overview on ATPase structure
- UniProt: P37808
- KEGG entry: [3]
- E.C. number: 3.6.3.14
Additional information
Expression and regulation
- Regulatory mechanism:
- Additional information:
- belongs to the 100 most abundant proteins PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Original publications
Hannes Hahne, Susanne Wolff, Michael Hecker, Dörte Becher
From complementarity to comprehensiveness--targeting the membrane proteome of growing Bacillus subtilis by divergent approaches.
Proteomics: 2008, 8(19);4123-36
[PubMed:18763711]
[WorldCat.org]
[DOI]
(I p)
Alain Lévine, Françoise Vannier, Cédric Absalon, Lauriane Kuhn, Peter Jackson, Elaine Scrivener, Valérie Labas, Joëlle Vinh, Patrick Courtney, Jérôme Garin, Simone J Séror
Analysis of the dynamic Bacillus subtilis Ser/Thr/Tyr phosphoproteome implicated in a wide variety of cellular processes.
Proteomics: 2006, 6(7);2157-73
[PubMed:16493705]
[WorldCat.org]
[DOI]
(P p)
Christine Eymann, Annette Dreisbach, Dirk Albrecht, Jörg Bernhardt, Dörte Becher, Sandy Gentner, Le Thi Tam, Knut Büttner, Gerrit Buurman, Christian Scharf, Simone Venz, Uwe Völker, Michael Hecker
A comprehensive proteome map of growing Bacillus subtilis cells.
Proteomics: 2004, 4(10);2849-76
[PubMed:15378759]
[WorldCat.org]
[DOI]
(P p)
M Santana, M S Ionescu, A Vertes, R Longin, F Kunst, A Danchin, P Glaser
Bacillus subtilis F0F1 ATPase: DNA sequence of the atp operon and characterization of atp mutants.
J Bacteriol: 1994, 176(22);6802-11
[PubMed:7961438]
[WorldCat.org]
[DOI]
(P p)