Difference between revisions of "Mdh"
| Line 44: | Line 44: | ||
{{SubtiWiki category|[[carbon core metabolism]]}}, | {{SubtiWiki category|[[carbon core metabolism]]}}, | ||
{{SubtiWiki category|[[membrane proteins]]}}, | {{SubtiWiki category|[[membrane proteins]]}}, | ||
| − | {{SubtiWiki category|[[phosphoproteins]]}} | + | {{SubtiWiki category|[[phosphoproteins]]}}, |
| + | [[most abundant proteins]] | ||
= This gene is a member of the following [[regulons]] = | = This gene is a member of the following [[regulons]] = | ||
| Line 141: | Line 142: | ||
* '''Additional information:''' | * '''Additional information:''' | ||
| + | ** belongs to the 100 [[most abundant proteins]] {{PubMed|15378759}} | ||
=Biological materials = | =Biological materials = | ||
| Line 174: | Line 176: | ||
=References= | =References= | ||
| − | <pubmed>10656796,18763711 14284712 922015 12100558 17218307 8550482 8045899 20933603 22517742 23136871 24325460 24571712</pubmed> | + | <pubmed>10656796,18763711 14284712 922015 12100558 17218307 8550482 8045899 20933603 22517742 23136871 24325460 15378759 24571712</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 13:00, 5 March 2014
- Description: malate dehydrogenase
| Gene name | mdh |
| Synonyms | citH |
| Essential | no |
| Product | malate dehydrogenase |
| Function | TCA cycle |
| Gene expression levels in SubtiExpress: mdh | |
| Interactions involving this protein in SubtInteract: Mdh | |
| Metabolic function and regulation of this protein in SubtiPathways: mdh | |
| MW, pI | 33 kDa, 4.727 |
| Gene length, protein length | 936 bp, 312 aa |
| Immediate neighbours | phoP, icd |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 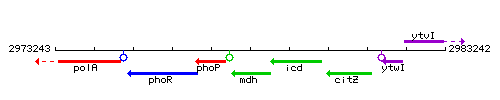
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
carbon core metabolism, membrane proteins, phosphoproteins, most abundant proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU29120
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: (S)-malate + NAD+ = oxaloacetate + NADH (according to Swiss-Prot)
- Protein family: MDH type 3 family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Cofactors: NAD+
- Effectors of protein activity:
- Localization:
- cytoplasm (according to Swiss-Prot), membrane associated PubMed
Database entries
- Structure: 1EMD (E.coli)
- UniProt: P49814
- KEGG entry: [3]
- E.C. number: 1.1.1.37
Additional information
- The enzyme is a tetramer PubMed
- extensive information on the structure and enzymatic properties of Mdh can be found at Proteopedia
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
- belongs to the 100 most abundant proteins PubMed
Biological materials
- Mutant:
- GP719(spc) & GP1150(spc), available in Jörg Stülke's lab
- GP790 (citZ-icd-mdh::kan), available in Jörg Stülke's lab
- Expression vector:
- pGP385: for expression, purification in E. coli with N-terminal His-tag, in pWH844, available in Jörg Stülke's lab
- pGP1123 (N-terminal Strep-tag, for SPINE, purification from B. subtilis, in pGP380) (available in Jörg Stülke's lab)
- pGP1755 (expression / purification of Mdh-S149A, with N-terminal His-tag from E. coli, in pWH844), available in Jörg Stülke's lab
- pGP1764 (for expression, purification in E. coli with N-terminal Strep-tag, in pGP172, available in Jörg Stülke's lab)
- GP1438(mdh-Strep (spc)) & GP1440(mdh-Strep (cat)), purification from B. subtilis, for SPINE, available in Jörg Stülke's lab
- lacZ fusion:
- GFP fusion: GP1431 (spc, based on pGP1870), available in Jörg Stülke's lab
- YFP fusion: GP1429 (spc, based on pGP1871), available in Jörg Stülke's lab
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Jörg Stülke's lab
- Antibody:
- FLAG-tag construct:
- GP1130 (spc, based on pGP1331), available in Jörg Stülke's lab
Labs working on this gene/protein
Your additional remarks
References