Difference between revisions of "RpmB"
| Line 60: | Line 60: | ||
=== Additional information=== | === Additional information=== | ||
| − | |||
| − | |||
| Line 78: | Line 76: | ||
* '''Kinetic information:''' | * '''Kinetic information:''' | ||
| − | * '''Domains:''' | + | * '''[[Domains]]:''' |
* '''Modification:''' | * '''Modification:''' | ||
| − | * ''' | + | * '''[[Cofactors]]:''' |
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| Line 101: | Line 99: | ||
=== Additional information=== | === Additional information=== | ||
| − | * the protein is significantly underrepresented in 45S assembly intermediates that accumulate upon depletion of [[RbgA]] {{PubMed|23700310}} | + | * the protein is significantly underrepresented in 45S assembly intermediates that accumulate upon depletion of [[RbgA]] {{PubMed|24335279,23700310}} |
=Expression and regulation= | =Expression and regulation= | ||
| Line 138: | Line 136: | ||
=References= | =References= | ||
| − | <pubmed>19653700 23002217,23033921 23700310</pubmed> | + | <pubmed>19653700 23002217,23033921 23700310 24335279</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 17:23, 18 December 2013
- Description: ribosomal protein
| Gene name | rpmB |
| Synonyms | yloT |
| Essential | no PubMed |
| Product | ribosomal protein L28 |
| Function | translation |
| Gene expression levels in SubtiExpress: rpmB | |
| Interactions involving this protein in SubtInteract: RpmB | |
| MW, pI | 6 kDa, 12.242 |
| Gene length, protein length | 186 bp, 62 aa |
| Immediate neighbours | spoVM, yloU |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed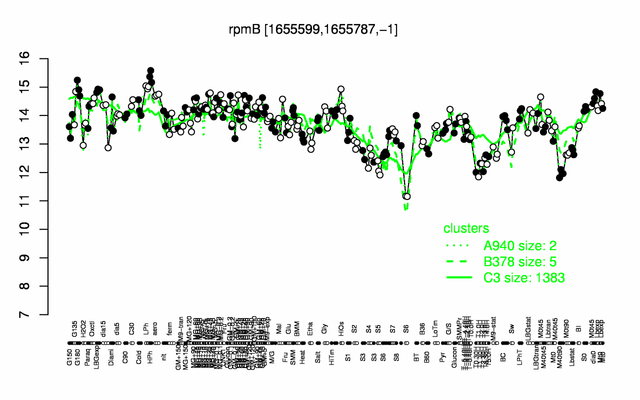
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU15820
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: ribosomal protein L28P family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity:
Database entries
- Structure:
- UniProt: P37807
- KEGG entry: [2]
- E.C. number:
Additional information
- the protein is significantly underrepresented in 45S assembly intermediates that accumulate upon depletion of RbgA PubMed
Expression and regulation
- Operon:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Ahmad Jomaa, Nikhil Jain, Joseph H Davis, James R Williamson, Robert A Britton, Joaquin Ortega
Functional domains of the 50S subunit mature late in the assembly process.
Nucleic Acids Res: 2014, 42(5);3419-35
[PubMed:24335279]
[WorldCat.org]
[DOI]
(I p)
Ningning Li, Yuling Chen, Qiang Guo, Yixiao Zhang, Yi Yuan, Chengying Ma, Haiteng Deng, Jianlin Lei, Ning Gao
Cryo-EM structures of the late-stage assembly intermediates of the bacterial 50S ribosomal subunit.
Nucleic Acids Res: 2013, 41(14);7073-83
[PubMed:23700310]
[WorldCat.org]
[DOI]
(I p)
Imke G de Jong, Jan-Willem Veening, Oscar P Kuipers
Single cell analysis of gene expression patterns during carbon starvation in Bacillus subtilis reveals large phenotypic variation.
Environ Microbiol: 2012, 14(12);3110-21
[PubMed:23033921]
[WorldCat.org]
[DOI]
(I p)
Genki Akanuma, Hideaki Nanamiya, Yousuke Natori, Koichi Yano, Shota Suzuki, Shuya Omata, Morio Ishizuka, Yasuhiko Sekine, Fujio Kawamura
Inactivation of ribosomal protein genes in Bacillus subtilis reveals importance of each ribosomal protein for cell proliferation and cell differentiation.
J Bacteriol: 2012, 194(22);6282-91
[PubMed:23002217]
[WorldCat.org]
[DOI]
(I p)
Matthew A Lauber, William E Running, James P Reilly
B. subtilis ribosomal proteins: structural homology and post-translational modifications.
J Proteome Res: 2009, 8(9);4193-206
[PubMed:19653700]
[WorldCat.org]
[DOI]
(P p)