Difference between revisions of "Icd"
(→References) |
|||
| Line 170: | Line 170: | ||
=References= | =References= | ||
| − | <pubmed>10656796,12850135 11290745 10348849 11751849 18763711 17726680 16493705 4147570 20389117 9244258 20933603 22517742</pubmed> | + | <pubmed>10656796,12850135 11290745 10348849 11751849 18763711 17726680 16493705 4147570 20389117 9244258 20933603 22517742 24325460 </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 13:44, 12 December 2013
- Description: isocitrate dehydrogenase
| Gene name | icd |
| Synonyms | citC |
| Essential | no |
| Product | isocitrate dehydrogenase |
| Function | TCA cycle |
| Gene expression levels in SubtiExpress: icd | |
| Interactions involving this protein in SubtInteract: Icd | |
| Metabolic function and regulation of this protein in SubtiPathways: Central C-metabolism | |
| MW, pI | 46 kDa, 4.833 |
| Gene length, protein length | 1269 bp, 423 aa |
| Immediate neighbours | mdh, citZ |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 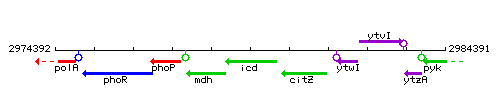
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
carbon core metabolism, membrane proteins, phosphoproteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU29130
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Isocitrate + NADP+ = 2-oxoglutarate + CO2 + NADPH (according to Swiss-Prot)
- Protein family: isocitrate and isopropylmalate dehydrogenases family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information: Reversible Michaelis-Menten PubMed
- Domains:
- Modification:
- Cofactor(s): Mg2+, Mn2+, NADP+
- Effectors of protein activity:
- Localization: attached to the membrane PubMed
Database entries
- Structure: 1HQS
- UniProt: P39126
- KEGG entry: [3]
- E.C. number: 1.1.1.42
Additional information
- This enzyme requires NADP+ exclusively. No activity was seen on the presence on NAD+ PubMed
- extensive information on the structure and enzymatic properties of Icd can be found at Proteopedia
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant: GP666 (spc), GP672 (erm), available in Jörg Stülke's lab
- Expression vector:
- pGP1121 (N-terminal Strep-tag, for SPINE, purification from B. subtilis, in pGP380) (available in Jörg Stülke's lab)
- lacZ fusion:
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Jörg Stülke's lab
- Antibody: available in Linc Sonenshein's lab
Labs working on this gene/protein
Linc Sonenshein, Tufts University, Boston, MA, USA Homepage
Your additional remarks
References