Difference between revisions of "CapA"
| Line 36: | Line 36: | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
| − | |||
| − | |||
| − | |||
| − | |||
<br/><br/> | <br/><br/> | ||
| Line 81: | Line 77: | ||
* '''Kinetic information:''' | * '''Kinetic information:''' | ||
| − | * '''Domains:''' | + | * '''[[Domains]]:''' |
* '''Modification:''' | * '''Modification:''' | ||
| − | * ''' | + | * '''[[Cofactors]]:''' |
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| Line 111: | Line 107: | ||
* '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=pgsA_3697821_3698963_-1 capA] {{PubMed|22383849}} | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=pgsA_3697821_3698963_-1 capA] {{PubMed|22383849}} | ||
| − | * '''Sigma factor:''' [[SigA]] [http://www.ncbi.nlm.nih.gov/sites/entrez/19420703 PubMed] | + | * '''[[Sigma factor]]:''' [[SigA]] [http://www.ncbi.nlm.nih.gov/sites/entrez/19420703 PubMed] |
* '''Regulation:''' | * '''Regulation:''' | ||
| Line 117: | Line 113: | ||
* '''Regulatory mechanism:''' activated by [[DegU]]-P [http://www.ncbi.nlm.nih.gov/sites/entrez/19420703 PubMed] | * '''Regulatory mechanism:''' activated by [[DegU]]-P [http://www.ncbi.nlm.nih.gov/sites/entrez/19420703 PubMed] | ||
| − | * '''Additional information:''' The operon is not expressed in the lab strain 168 due to lack of expression of [[DegQ]] resulting in inactivity of [[DegS]] and [[DegU]] {{PubMed|21965392}} | + | * '''Additional information:''' |
| + | ** The operon is not expressed in the lab strain 168 due to lack of expression of [[DegQ]] resulting in inactivity of [[DegS]] and [[DegU]] {{PubMed|21965392}} | ||
| + | ** expression of the operon is reduced in ''[[motA]]'' or ''[[motB]]'' mutants due to reduced [[DegU]] phosphorylation {{PubMed|24296669}} | ||
=Biological materials = | =Biological materials = | ||
| Line 142: | Line 140: | ||
==Original publications== | ==Original publications== | ||
'''Additional publications:''' {{PubMed|21965392}} | '''Additional publications:''' {{PubMed|21965392}} | ||
| − | <pubmed>11751809,19420703, 16233197 20564357 20564574 </pubmed> | + | <pubmed>11751809,19420703, 16233197 20564357 20564574 24296669 21965392</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 20:14, 4 December 2013
- Description: similar to capsular polyglutamate biosynthesis
| Gene name | capA |
| Synonyms | ywtB, pgsA |
| Essential | no |
| Product | unknown |
| Function | unknown |
| Gene expression levels in SubtiExpress: capA | |
| MW, pI | 42 kDa, 9.274 |
| Gene length, protein length | 1140 bp, 380 aa |
| Immediate neighbours | capE, capC |
| Gene sequence (+200bp) | Protein sequence |
Genetic context 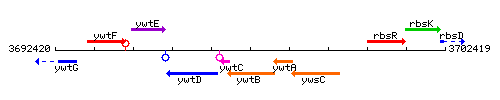
This image was kindly provided by SubtiList
| |
| Expression at a glance PubMed 500px | |
Contents
Categories containing this gene/protein
capsule biosynthesis and degradation
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU35880
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity:
Database entries
- Structure:
- UniProt: P96738
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Original publications
Additional publications: PubMed