Difference between revisions of "DefA"
| Line 4: | Line 4: | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Gene name''' | |style="background:#ABCDEF;" align="center"|'''Gene name''' | ||
| − | |'' | + | |''defA'' |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Synonyms''' || ''yloK '' | + | |style="background:#ABCDEF;" align="center"| '''Synonyms''' || ''yloK, def '' |
|- | |- | ||
|style="background:#ABCDEF;" align="center"| '''Essential''' || no | |style="background:#ABCDEF;" align="center"| '''Essential''' || no | ||
| Line 12: | Line 12: | ||
|style="background:#ABCDEF;" align="center"| '''Product''' || formylmethionine deformylase | |style="background:#ABCDEF;" align="center"| '''Product''' || formylmethionine deformylase | ||
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"|'''Function''' || translation | + | |style="background:#ABCDEF;" align="center"|'''Function''' || [[translation]] |
|- | |- | ||
| − | |colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http://subtiwiki.uni-goettingen.de/apps/expression/ ''Subti''Express]''': [http://subtiwiki.uni-goettingen.de/apps/expression/expression.php?search=BSU15720 | + | |colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http://subtiwiki.uni-goettingen.de/apps/expression/ ''Subti''Express]''': [http://subtiwiki.uni-goettingen.de/apps/expression/expression.php?search=BSU15720 defA] |
|- | |- | ||
|style="background:#ABCDEF;" align="center"| '''MW, pI''' || 17 kDa, 4.731 | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || 17 kDa, 4.731 | ||
| Line 35: | Line 35: | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
| − | |||
| − | |||
| − | |||
| − | |||
<br/><br/> | <br/><br/> | ||
| Line 107: | Line 103: | ||
=Expression and regulation= | =Expression and regulation= | ||
| − | * '''Operon:''' ''[[ | + | * '''Operon:''' ''[[defA]]-[[fmt]]-[[yloM]]'' {{PubMed|16964327}} |
| − | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=defA_1646512_1646994_1 | + | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=defA_1646512_1646994_1 defA] {{PubMed|22383849}} |
| − | * '''Sigma factor:''' [[SigA]] [ | + | * '''[[Sigma factor]]:''' [[SigA]] ''[[defA]]-[[fmt]]-[[yloM]]'' {{PubMed|16964327}} |
* '''Regulation:''' | * '''Regulation:''' | ||
Revision as of 10:58, 18 August 2013
- Description: formylmethionine deformylase
| Gene name | defA |
| Synonyms | yloK, def |
| Essential | no |
| Product | formylmethionine deformylase |
| Function | translation |
| Gene expression levels in SubtiExpress: defA | |
| MW, pI | 17 kDa, 4.731 |
| Gene length, protein length | 480 bp, 160 aa |
| Immediate neighbours | priA, fmt |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 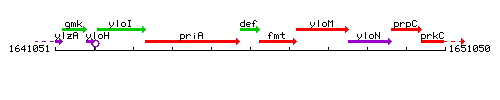
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
translation, protein modification
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU15720
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Formyl-L-methionyl peptide + H2O = formate + methionyl peptide (according to Swiss-Prot)
- Protein family: polypeptide deformylase family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure:
- UniProt: P94462
- KEGG entry: [2]
- E.C. number: 3.5.1.88
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Additional publications: PubMed