Difference between revisions of "CwlO"
(→Categories containing this gene/protein) |
|||
| Line 52: | Line 52: | ||
===Phenotypes of a mutant === | ===Phenotypes of a mutant === | ||
* a ''[[cwlO]] [[lytE]]'' mutant is not viable {{PubMed|17581128,22139507}} | * a ''[[cwlO]] [[lytE]]'' mutant is not viable {{PubMed|17581128,22139507}} | ||
| − | * shorter, fatter cells {{PubMed| | + | * shorter, fatter cells {{PubMed|23855774}} |
=== Database entries === | === Database entries === | ||
| Line 85: | Line 85: | ||
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| − | ** [[CwlO]] requires activation by [[FtsE]]-[[FtsX]] {{PubMed| | + | ** [[CwlO]] requires activation by [[FtsE]]-[[FtsX]] {{PubMed|23855774}} |
* '''[[SubtInteract|Interactions]]:''' | * '''[[SubtInteract|Interactions]]:''' | ||
| Line 140: | Line 140: | ||
=References= | =References= | ||
== Reviews == | == Reviews == | ||
| − | + | <pubmed>23066944</pubmed> | |
| − | <pubmed></pubmed> | ||
== Original publications == | == Original publications == | ||
| − | + | <pubmed>21478646 16233686,17581128, 20525796,18957862, 20059685 ,22139507 23855774</pubmed> | |
| − | <pubmed>16233686,17581128, 20525796,18957862, 20059685 ,22139507</pubmed> | ||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 16:53, 19 July 2013
- Description: D,L-endopeptidase-type autolysin, primary autolytic pathway for cell elongation
| Gene name | cwlO |
| Synonyms | yzkA, yvcE |
| Essential | no |
| Product | endopeptidase-type autolysin |
| Function | cell wall synthesis, cell proliferation |
| Gene expression levels in SubtiExpress: cwlO | |
| MW, pI | 50 kDa, 5.326 |
| Gene length, protein length | 1419 bp, 473 aa |
| Immediate neighbours | trxB, yvcD |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 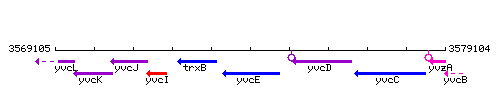
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
cell wall synthesis, cell wall degradation/ turnover
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU34800
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: peptidase C40 family (according to Swiss-Prot)
- Paralogous protein(s): the C-terminal D,L-endopeptidase domains of LytE, LytF, CwlS, and CwlO exhibit strong sequence similarity
Extended information on the protein
- Kinetic information:
- Domains:
- C-terminal D,L-endopeptidase domain PubMed
- Modification:
- Cofactor(s):
Database entries
- Structure:
- UniProt: P40767
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Regulatory mechanism:
- Additional information:
- The mRNA has a long 5' leader region. This may indicate RNA-based regulation PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Original publications