Difference between revisions of "LysS"
m (Reverted edits by 134.76.70.252 (talk) to last revision by Jstuelk) |
|||
| Line 1: | Line 1: | ||
| − | + | * '''Description:''' lysyl-tRNA synthetase <br/><br/> | |
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
| Line 13: | Line 13: | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Function''' || translation | |style="background:#ABCDEF;" align="center"|'''Function''' || translation | ||
| − | |||
| − | |||
|- | |- | ||
|colspan="2" style="background:#FAF8CC;" align="center"| '''Metabolic function and regulation of this protein in [[SubtiPathways|''Subti''Pathways]]: <br/>[http://subtiwiki.uni-goettingen.de/pathways/tRNA_charging.html tRNA charging], [http://subtiwiki.uni-goettingen.de/pathways/folate_biosynthesis.html Folate]''' | |colspan="2" style="background:#FAF8CC;" align="center"| '''Metabolic function and regulation of this protein in [[SubtiPathways|''Subti''Pathways]]: <br/>[http://subtiwiki.uni-goettingen.de/pathways/tRNA_charging.html tRNA charging], [http://subtiwiki.uni-goettingen.de/pathways/folate_biosynthesis.html Folate]''' | ||
| Line 28: | Line 26: | ||
|colspan="2" | '''Genetic context''' <br/> [[Image:lysS_context.gif]] | |colspan="2" | '''Genetic context''' <br/> [[Image:lysS_context.gif]] | ||
<div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | <div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | ||
| − | |||
| − | |||
|- | |- | ||
|} | |} | ||
__TOC__ | __TOC__ | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
<br/><br/><br/><br/><br/><br/> | <br/><br/><br/><br/><br/><br/> | ||
| Line 116: | Line 106: | ||
''lysS'' [http://www.ncbi.nlm.nih.gov/sites/entrez/9084182 PubMed] | ''lysS'' [http://www.ncbi.nlm.nih.gov/sites/entrez/9084182 PubMed] | ||
| − | * | + | * '''[[Sigma factor]]:''' ''[[pabB]]'': [[SigA]] [http://www.ncbi.nlm.nih.gov/sites/entrez/9084182 PubMed] |
| − | |||
| − | |||
* '''Regulation:''' | * '''Regulation:''' | ||
Revision as of 10:21, 7 August 2012
- Description: lysyl-tRNA synthetase
| Gene name | lysS |
| Synonyms | |
| Essential | yes PubMed |
| Product | lysyl-tRNA synthetase |
| Function | translation |
| Metabolic function and regulation of this protein in SubtiPathways: tRNA charging, Folate | |
| MW, pI | 57 kDa, 5.034 |
| Gene length, protein length | 1497 bp, 499 aa |
| Immediate neighbours | yacF, rrnJ-16S |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 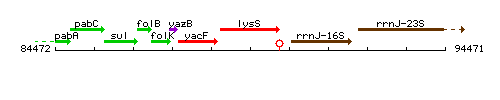
This image was kindly provided by SubtiList
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU00820
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: ATP + L-lysine + tRNA(Lys) = AMP + diphosphate + L-lysyl-tRNA(Lys) (according to Swiss-Prot)
- Protein family: class-II aminoacyl-tRNA synthetase family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization:
- cytoplasm (according to Swiss-Prot)
Database entries
- Structure: 2E9I (from Geobacillus stearothermophilus, in complex with L-Lysine hydroxamate-AMP) PubMed
- UniProt: P37477
- KEGG entry: [3]
- E.C. number: 6.1.1.6
Additional information
Expression and regulation
lysS PubMed
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References