Difference between revisions of "NagZ"
| Line 1: | Line 1: | ||
| − | + | ||
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
| Line 13: | Line 13: | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Function''' || cell wall recycling | |style="background:#ABCDEF;" align="center"|'''Function''' || cell wall recycling | ||
| + | |- | ||
| + | |colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http://cellpublisher.gobics.de/subtiexpress/ ''Subti''Express]''': [http://cellpublisher.gobics.de/subtiexpress/bsu/BSU01660 nagZ] | ||
|- | |- | ||
|colspan="2" style="background:#FAF8CC;" align="center"| '''Metabolic function and regulation of this protein in [[SubtiPathways|''Subti''Pathways]]: <br/>[http://subtiwiki.uni-goettingen.de/pathways/murein/index.html Murein recycling]''' | |colspan="2" style="background:#FAF8CC;" align="center"| '''Metabolic function and regulation of this protein in [[SubtiPathways|''Subti''Pathways]]: <br/>[http://subtiwiki.uni-goettingen.de/pathways/murein/index.html Murein recycling]''' | ||
Revision as of 14:31, 6 August 2012
| Gene name | nagZ |
| Synonyms | yzbA, ybbD |
| Essential | no |
| Product | N-acetylglucosaminidase |
| Function | cell wall recycling |
| Gene expression levels in SubtiExpress: nagZ | |
| Metabolic function and regulation of this protein in SubtiPathways: Murein recycling | |
| MW, pI | 70 kDa, 9.76 |
| Gene length, protein length | 1926 bp, 642 aa |
| Immediate neighbours | ybbC, amiE |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 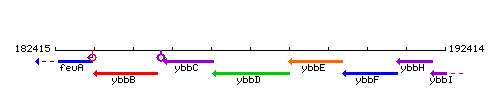
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed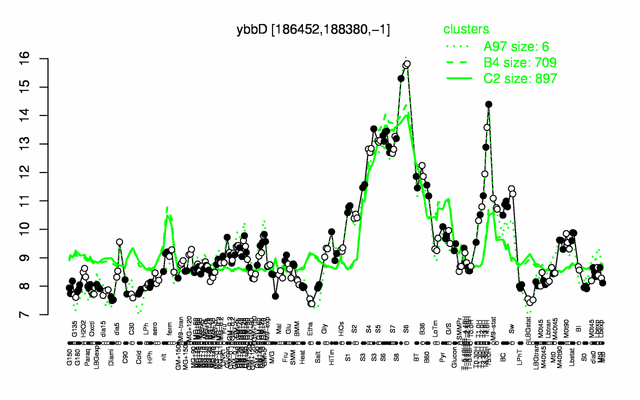
| |
Contents
Categories containing this gene/protein
cell wall degradation/ turnover
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU01660
Phenotypes of a mutant
increased autolysis PubMed
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: cleaves muropeptides derived from peptidoglycan, but not peptidoglycan itself PubMed
- Protein family: glycosyl hydrolase 3 family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization:
- secreted (with signal peptide), remains to some extent cell wall-associated PubMed
Database entries
- UniProt: P40406
- KEGG entry: [2]
- E.C. number:
Additional information
The gene is mis-annotated in KEGG as an ortholog of beta-N-acetylhexosaminidase EC 3.2.1.52. It is marked in MetaCyc as “similar to beta-hexosaminidase”. No EC annotation is available in Swiss-ProtSwiss-Prot.supporting the annotation is available. PubMed
Expression and regulation
- Sigma factor:
- Regulation:
- expressed in late exponential and early stationary phase PubMed
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant: available in Christoph Mayer's lab PubMed
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Additional references: PubMed
Pierre Nicolas, Ulrike Mäder, Etienne Dervyn, Tatiana Rochat, Aurélie Leduc, Nathalie Pigeonneau, Elena Bidnenko, Elodie Marchadier, Mark Hoebeke, Stéphane Aymerich, Dörte Becher, Paola Bisicchia, Eric Botella, Olivier Delumeau, Geoff Doherty, Emma L Denham, Mark J Fogg, Vincent Fromion, Anne Goelzer, Annette Hansen, Elisabeth Härtig, Colin R Harwood, Georg Homuth, Hanne Jarmer, Matthieu Jules, Edda Klipp, Ludovic Le Chat, François Lecointe, Peter Lewis, Wolfram Liebermeister, Anika March, Ruben A T Mars, Priyanka Nannapaneni, David Noone, Susanne Pohl, Bernd Rinn, Frank Rügheimer, Praveen K Sappa, Franck Samson, Marc Schaffer, Benno Schwikowski, Leif Steil, Jörg Stülke, Thomas Wiegert, Kevin M Devine, Anthony J Wilkinson, Jan Maarten van Dijl, Michael Hecker, Uwe Völker, Philippe Bessières, Philippe Noirot
Condition-dependent transcriptome reveals high-level regulatory architecture in Bacillus subtilis.
Science: 2012, 335(6072);1103-6
[PubMed:22383849]
[WorldCat.org]
[DOI]
(I p)
Silke Litzinger, Amanda Duckworth, Katja Nitzsche, Christian Risinger, Valentin Wittmann, Christoph Mayer
Muropeptide rescue in Bacillus subtilis involves sequential hydrolysis by beta-N-acetylglucosaminidase and N-acetylmuramyl-L-alanine amidase.
J Bacteriol: 2010, 192(12);3132-43
[PubMed:20400549]
[WorldCat.org]
[DOI]
(I p)
Jonathan Reizer, Steffi Bachem, Aiala Reizer, Maryvonne Arnaud, Milton H Saier, Jörg Stülke
Novel phosphotransferase system genes revealed by genome analysis - the complete complement of PTS proteins encoded within the genome of Bacillus subtilis.
Microbiology (Reading): 1999, 145 ( Pt 12);3419-3429
[PubMed:10627040]
[WorldCat.org]
[DOI]
(P p)
R C Berkeley, S J Brewer, J M Ortiz, J B Gillespie
An exo- -N-acetylglucosaminidase from Bacillus subtilis B; characterization.
Biochim Biophys Acta: 1973, 309(1);157-68
[PubMed:4196675]
[WorldCat.org]
[DOI]
(P p)
J M Ortiz, J B Gillespie, R C Berkeley
An exo- -N-acetylglucosaminidase from Bacillus subtilis B; extraction and purification.
Biochim Biophys Acta: 1972, 289(1);174-86
[PubMed:4628806]
[WorldCat.org]
[DOI]
(P p)