Difference between revisions of "BdbD"
| Line 1: | Line 1: | ||
| − | * '''Description:''' thiol-disulfide oxidoreductase, required for the formation of thiol disulfide bonds in | + | * '''Description:''' thiol-disulfide oxidoreductase, required for the formation of thiol disulfide bonds in several proteins <br/><br/> |
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
| Line 12: | Line 12: | ||
|style="background:#ABCDEF;" align="center"| '''Product''' || thiol-disulfide oxidoreductase | |style="background:#ABCDEF;" align="center"| '''Product''' || thiol-disulfide oxidoreductase | ||
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"|'''Function''' || | + | |style="background:#ABCDEF;" align="center"|'''Function''' || oxidative folding of proteins |
|- | |- | ||
|colspan="2" style="background:#FAF8CC;" align="center"| '''Metabolic function and regulation of this protein in [[SubtiPathways|''Subti''Pathways]]: <br/>[http://subtiwiki.uni-goettingen.de/wiki/index.php/Protein_secretion Protein secretion]''' | |colspan="2" style="background:#FAF8CC;" align="center"| '''Metabolic function and regulation of this protein in [[SubtiPathways|''Subti''Pathways]]: <br/>[http://subtiwiki.uni-goettingen.de/wiki/index.php/Protein_secretion Protein secretion]''' | ||
| Line 58: | Line 58: | ||
===Phenotypes of a mutant === | ===Phenotypes of a mutant === | ||
| − | + | * loss of transformability [http://www.ncbi.nlm.nih.gov/sites/entrez/11744713 PubMed] | |
| − | loss of transformability [http://www.ncbi.nlm.nih.gov/sites/entrez/11744713 PubMed] | + | * sensitive to osmotic shock {{PubMed|22540663}} |
| + | * several proteins are absent from the membrane proteome of a ''[[bdbC]]-[[bdbD]]'' mutant: {{PubMed|22540663}} | ||
| + | ** the membrane proteins [[BglP]], [[TcyP]], [[SipU]], [[LytA]], and [[YxaI]] {{PubMed|22540663}} | ||
| + | ** the cytoplasmic or membrane-associated proteins [[GlkX]], [[PyrAA]], [[PyrAB]], [[PyrH]], [[PyrE]], [[PyrF]], [[DegS]], [[YbxA]] {{PubMed|22540663}} | ||
=== Database entries === | === Database entries === | ||
| Line 68: | Line 71: | ||
=== Additional information=== | === Additional information=== | ||
| − | |||
| − | |||
| − | |||
=The protein= | =The protein= | ||
| Line 76: | Line 76: | ||
=== Basic information/ Evolution === | === Basic information/ Evolution === | ||
| − | * '''Catalyzed reaction/ biological activity:''' formation of thiol disulfide bonds in [[ComGC]] [http://www.ncbi.nlm.nih.gov/sites/entrez/11744713 PubMed] | + | * '''Catalyzed reaction/ biological activity:''' |
| + | ** formation of thiol disulfide bonds in [[ComEC]] and [[ComGC]] (together with [[BdbC]]) [http://www.ncbi.nlm.nih.gov/sites/entrez/11744713 PubMed] | ||
* '''Protein family:''' DsbA subfamily (according to Swiss-Prot) | * '''Protein family:''' DsbA subfamily (according to Swiss-Prot) | ||
| Line 145: | Line 146: | ||
=References= | =References= | ||
| − | <pubmed>19535335, 11744713,15661011,11872755,16751195,11844773, 12480901</pubmed> | + | <pubmed>19535335, 11744713,15661011,11872755,16751195,11844773, 12480901 22540663 </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 14:35, 6 May 2012
- Description: thiol-disulfide oxidoreductase, required for the formation of thiol disulfide bonds in several proteins
| Gene name | bdbD |
| Synonyms | yvgV |
| Essential | no |
| Product | thiol-disulfide oxidoreductase |
| Function | oxidative folding of proteins |
| Metabolic function and regulation of this protein in SubtiPathways: Protein secretion | |
| MW, pI | 24 kDa, 5.089 |
| Gene length, protein length | 666 bp, 222 aa |
| Immediate neighbours | bdbC, cadA |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 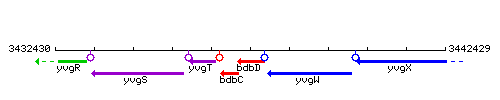
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
genetic competence, chaperones/ protein folding, sporulation proteins, membrane proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU33480
Phenotypes of a mutant
- loss of transformability PubMed
- sensitive to osmotic shock PubMed
- several proteins are absent from the membrane proteome of a bdbC-bdbD mutant: PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: DsbA subfamily (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s): Ca (2+) PubMed
- Effectors of protein activity:
- Localization: membrane, faced to the outer side of the membrane PubMed
Database entries
- UniProt: O32218
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References