Difference between revisions of "GlnA"
(→Database entries) |
|||
| Line 104: | Line 104: | ||
* '''Structure:''' | * '''Structure:''' | ||
** [http://www.rcsb.org/pdb/explore/explore.do?structureId=3QAJ 3QAJ] (complex with ATP) | ** [http://www.rcsb.org/pdb/explore/explore.do?structureId=3QAJ 3QAJ] (complex with ATP) | ||
| − | ** [http://www.rcsb.org/pdb/101/motm.do?momID=30 | + | ** [http://www.rcsb.org/pdb/101/motm.do?momID=30 A general discussion of GS structure] |
* '''UniProt:''' [http://www.uniprot.org/uniprot/P12425 P12425] | * '''UniProt:''' [http://www.uniprot.org/uniprot/P12425 P12425] | ||
Revision as of 17:38, 4 May 2012
- Description: trigger enzyme: glutamine synthetase and effector of TnrA and GlnR
| Gene name | glnA |
| Synonyms | |
| Essential | no |
| Product | trigger enzyme: glutamine synthetase |
| Function | glutamine biosynthesis, control of TnrA and GlnR activity |
| Interactions involving this protein in SubtInteract: GlnA | |
| Metabolic function and regulation of this protein in SubtiPathways: Ammonium/ glutamate | |
| MW, pI | 50 kDa, 4.874 |
| Gene length, protein length | 1332 bp, 444 aa |
| Immediate neighbours | glnR, ynxB |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 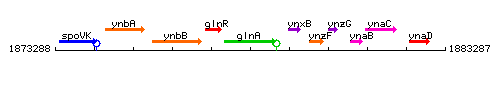
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
biosynthesis/ acquisition of amino acids, transcription factors and their control, trigger enzyme, phosphoproteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU17460
Phenotypes of a mutant
auxotrophic for glutamine
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: ATP + L-glutamate + NH3 = ADP + phosphate + L-glutamine (according to Swiss-Prot)
- Protein family: glutamine synthetase family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information: K(M) for: Glu: 27 mM, ATP: 2.4 mM, ammonium: 0.18 mM; v(max): 3.7 µmol/min/mg
- Domains: glutamate binding flap (aa 300 ... 306: protects unstable intermediates from abberant hydrolysis)
- Modification: phosphorylated on ser/ thr/ tyr PubMed, in vitro phosphorylated by PrkC on Thr-26, Thr-147, Ser-207, and Thr-286 PubMed
- Cofactor(s): Mg(2+)
- Effectors of protein activity: feedback inhibition by glutamine, glutamine binds the entrance site for glutamate
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- Structure:
- 3QAJ (complex with ATP)
- A general discussion of GS structure
- UniProt: P12425
- KEGG entry: [3]
- E.C. number: 6.3.1.2
Additional information
GlnA is a homooligomer of 12 subunits
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant: GP247 (cat), available in Stülke lab
- Expression vector:
- GFP fusion:
- two-hybrid system:
- Antibody: available in Karl Forchhammer lab
Labs working on this gene/protein
Susan Fisher, Boston, USA homepage
Your additional remarks
References
Reviews
Fabian M Commichau, Jörg Stülke
Trigger enzymes: bifunctional proteins active in metabolism and in controlling gene expression.
Mol Microbiol: 2008, 67(4);692-702
[PubMed:18086213]
[WorldCat.org]
[DOI]
(P p)
S H Fisher
Regulation of nitrogen metabolism in Bacillus subtilis: vive la différence!
Mol Microbiol: 1999, 32(2);223-32
[PubMed:10231480]
[WorldCat.org]
[DOI]
(P p)
Original publications
Additional publications: PubMed