Difference between revisions of "SirA"
| Line 26: | Line 26: | ||
|colspan="2" | '''Genetic context''' <br/> [[Image:yneE_context.gif]] | |colspan="2" | '''Genetic context''' <br/> [[Image:yneE_context.gif]] | ||
<div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | <div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | ||
| + | |- | ||
| + | |colspan="2" |'''[http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=yneE_1922017_1922463_1 Expression at a glance]'''   {{PubMed|22383849}}<br/>[[Image:sirA_expression.png|500px]] | ||
|- | |- | ||
|} | |} | ||
__TOC__ | __TOC__ | ||
| + | <br/><br/><br/><br/> | ||
| + | <br/><br/><br/><br/> | ||
| + | <br/><br/><br/><br/> | ||
| + | <br/><br/><br/><br/> | ||
| + | <br/><br/><br/><br/> | ||
| + | |||
<br/><br/> | <br/><br/> | ||
Revision as of 10:24, 19 April 2012
- Description: sporulation protein, inhibits DNA replication
| Gene name | sirA |
| Synonyms | yneE, yoxF |
| Essential | no |
| Product | inhibitor of DNA replication |
| Function | control of chromosome copy number |
| Interactions involving this protein in SubtInteract: SirA | |
| MW, pI | 17 kDa, 7.353 |
| Gene length, protein length | 444 bp, 148 aa |
| Immediate neighbours | tkt, yneF |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 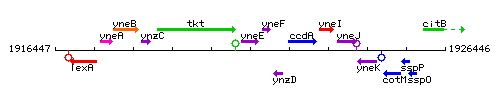
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
yneE was renamed to sirA.
Basic information
- Locus tag: BSU17900
Phenotypes of a mutant
"over-replication" during sporulation PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: interacts with DnaA and displaces it from the replication origin, resulting in inhibition of replication PubMed
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure:
- UniProt: P45707
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Sigma factor:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
sirA (sporulation inhibitor of replication) is activated by Spo0A~P and inhibits DNA replication during sporulation.
References
Reviews
Original publications
Lilah Rahn-Lee, Houra Merrikh, Alan D Grossman, Richard Losick
The sporulation protein SirA inhibits the binding of DnaA to the origin of replication by contacting a patch of clustered amino acids.
J Bacteriol: 2011, 193(6);1302-7
[PubMed:21239581]
[WorldCat.org]
[DOI]
(I p)
Jennifer K Wagner, Kathleen A Marquis, David Z Rudner
SirA enforces diploidy by inhibiting the replication initiator DnaA during spore formation in Bacillus subtilis.
Mol Microbiol: 2009, 73(5);963-74
[PubMed:19682252]
[WorldCat.org]
[DOI]
(I p)
Lilah Rahn-Lee, Boris Gorbatyuk, Ole Skovgaard, Richard Losick
The conserved sporulation protein YneE inhibits DNA replication in Bacillus subtilis.
J Bacteriol: 2009, 191(11);3736-9
[PubMed:19329632]
[WorldCat.org]
[DOI]
(I p)
Virginie Molle, Masaya Fujita, Shane T Jensen, Patrick Eichenberger, José E González-Pastor, Jun S Liu, Richard Losick
The Spo0A regulon of Bacillus subtilis.
Mol Microbiol: 2003, 50(5);1683-701
[PubMed:14651647]
[WorldCat.org]
[DOI]
(P p)
T Schiött, C von Wachenfeldt, L Hederstedt
Identification and characterization of the ccdA gene, required for cytochrome c synthesis in Bacillus subtilis.
J Bacteriol: 1997, 179(6);1962-73
[PubMed:9068642]
[WorldCat.org]
[DOI]
(P p)