Difference between revisions of "RplK"
m (Reverted edits by 134.76.70.252 (talk) to last revision by Jstuelk) |
|||
| Line 102: | Line 102: | ||
* '''Operon:''' | * '''Operon:''' | ||
| + | |||
| + | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=rplK_118591_119016_1 rplK] {{PubMed|22383849}} | ||
* '''Sigma factor:''' | * '''Sigma factor:''' | ||
Revision as of 11:13, 12 April 2012
- Description: ribosomal protein
| Gene name | rplK |
| Synonyms | relC, tsp |
| Essential | no |
| Product | ribosomal protein L11 (BL11) |
| Function | translation |
| Interactions involving this protein in SubtInteract: RplK | |
| MW, pI | 14 kDa, 9.718 |
| Gene length, protein length | 423 bp, 141 aa |
| Immediate neighbours | nusG, rplA |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 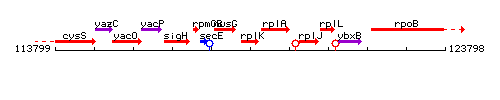
This image was kindly provided by SubtiList
| |
Contents
Categories containing this gene/protein
translation, universally conserved proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU01020
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: ribosomal protein L11P family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure: 1FOX (C-terminal domain, Geobacillus stearothermophilus), 2FOW (RNA binding domain in complex with RNA, Geobacillus stearothermophilus)
- UniProt: Q06796
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Sigma factor:
- Regulatory mechanism:
- Additional information:
- The mRNA has a long 5' leader region. This may indicate RNA-based regulation PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Irnov Irnov, Cynthia M Sharma, Jörg Vogel, Wade C Winkler
Identification of regulatory RNAs in Bacillus subtilis.
Nucleic Acids Res: 2010, 38(19);6637-51
[PubMed:20525796]
[WorldCat.org]
[DOI]
(I p)
Sascha Baumann, Sebastian Schoof, Marcel Bolten, Claudia Haering, Motoki Takagi, Kazuo Shin-ya, Hans-Dieter Arndt
Molecular determinants of microbial resistance to thiopeptide antibiotics.
J Am Chem Soc: 2010, 132(20);6973-81
[PubMed:20441189]
[WorldCat.org]
[DOI]
(I p)
Matthew A Lauber, William E Running, James P Reilly
B. subtilis ribosomal proteins: structural homology and post-translational modifications.
J Proteome Res: 2009, 8(9);4193-206
[PubMed:19653700]
[WorldCat.org]
[DOI]
(P p)
Christine Eymann, Georg Homuth, Christian Scharf, Michael Hecker
Bacillus subtilis functional genomics: global characterization of the stringent response by proteome and transcriptome analysis.
J Bacteriol: 2002, 184(9);2500-20
[PubMed:11948165]
[WorldCat.org]
[DOI]
(P p)
S Zhang, J M Scott, W G Haldenwang
Loss of ribosomal protein L11 blocks stress activation of the Bacillus subtilis transcription factor sigma(B).
J Bacteriol: 2001, 183(7);2316-21
[PubMed:11244072]
[WorldCat.org]
[DOI]
(P p)