Difference between revisions of "LutB"
(→Expression and regulation) |
|||
| Line 110: | Line 110: | ||
** induction by lactate [http://www.ncbi.nlm.nih.gov/sites/entrez/19201793 PubMed] | ** induction by lactate [http://www.ncbi.nlm.nih.gov/sites/entrez/19201793 PubMed] | ||
** less expressed under conditions of extreme iron limitation ([[FsrA]]) [http://www.ncbi.nlm.nih.gov/pubmed/18697947 PubMed] | ** less expressed under conditions of extreme iron limitation ([[FsrA]]) [http://www.ncbi.nlm.nih.gov/pubmed/18697947 PubMed] | ||
| − | ** part of the iron sparing response ([[FsrA]]) {{PubMed| | + | ** part of the iron sparing response, down-regulation in a'' [[fur]]'' mutant ([[Fur]], [[FsrA]], [[FpbB]]) {{PubMed|22427629}} |
* '''Regulatory mechanism:''' | * '''Regulatory mechanism:''' | ||
** [[SinR]]: transcription repression [http://www.ncbi.nlm.nih.gov/sites/entrez/19201793 PubMed] | ** [[SinR]]: transcription repression [http://www.ncbi.nlm.nih.gov/sites/entrez/19201793 PubMed] | ||
** dual repression by [[LutR]] (inducer lactate) and [[SinR]] [http://www.ncbi.nlm.nih.gov/sites/entrez/19201793 PubMed] | ** dual repression by [[LutR]] (inducer lactate) and [[SinR]] [http://www.ncbi.nlm.nih.gov/sites/entrez/19201793 PubMed] | ||
| − | ** [[FsrA]]: translational repression {{PubMed|18697947}} | + | ** [[FsrA]]: translational repression {{PubMed|22427629,18697947}} |
| + | ** [[FbpB]]: acts as RNA chaperone, increases interaction between ''[[fsrA]]'' and ''[[lutA]]-[[lutB]]-[[lutC]]'' RNAs {{PubMed|22427629}} | ||
* '''Additional information:''' | * '''Additional information:''' | ||
Revision as of 15:26, 25 March 2012
- Description: lactate catabolic enzyme
| Gene name | lutB |
| Synonyms | yvfW |
| Essential | no |
| Product | lactate oxidase |
| Function | lactate utilization |
| Metabolic function and regulation of this protein in SubtiPathways: Central C-metabolism | |
| MW, pI | 53 kDa, 5.848 |
| Gene length, protein length | 1437 bp, 479 aa |
| Immediate neighbours | lutC, lutA |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 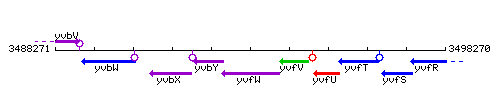
This image was kindly provided by SubtiList
| |
Contents
Categories containing this gene/protein
utilization of specific carbon sources
This gene is a member of the following regulons
FsrA regulon, LutR regulon, SinR regulon
The gene
Basic information
- Locus tag: BSU34040
Phenotypes of a mutant
no growth with lactate as the single carbon source PubMed
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: oxidation of lactate to pyruvate PubMed
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s): contains an iron-sulfur cluster
- Effectors of protein activity:
Database entries
- Structure:
- UniProt: O07021
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Richard Losick, Harvard Univ., Cambridge, USA homepage
Your additional remarks
References