Difference between revisions of "Nos"
(→References) |
|||
| Line 127: | Line 127: | ||
=References= | =References= | ||
| − | <pubmed>12220171,11856757,20006999 , </pubmed> | + | <pubmed>12220171,11856757,20006999 21310962 , </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 13:48, 12 February 2011
- Description: nitric-oxide synthase
| Gene name | nos |
| Synonyms | yflM |
| Essential | no |
| Product | nitric-oxide synthase |
| Function | production of nitric oxide |
| MW, pI | 38 kDa, 5.528 |
| Gene length, protein length | 1008 bp, 336 aa |
| Immediate neighbours | yflN, yflL |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 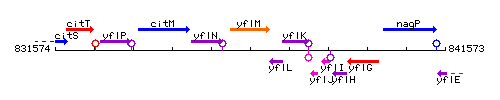
This image was kindly provided by SubtiList
| |
Contents
Categories containing this gene/protein
miscellaneous metabolic pathways, biosynthesis of antibacterial compounds
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU07630
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: Bacterial NOS oxygenase subfamily (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s): heme PubMed
- Effectors of protein activity:
- Interactions:
- Localization:
Database entries
- UniProt: O34453
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information: Note that the gene yflL is located between nos and yflK, but on the opposite strand.
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References