Difference between revisions of "MreB"
| Line 130: | Line 130: | ||
<pubmed>17506674 17158703 15037301 </pubmed> | <pubmed>17506674 17158703 15037301 </pubmed> | ||
==Loclization== | ==Loclization== | ||
| − | <pubmed> 20566861 </pubmed> | + | <pubmed> 17064365, 20566861 </pubmed> |
| − | == | + | ==Other original publications== |
| − | <pubmed>15752190,11290328,12809607,1400224,19192185,7836311, 16950129 | + | <pubmed>15752190,11290328,12809607,1400224,19192185,7836311, 16950129, 19192185, 19117023 19643765 19654094 19659933 8459776 11544518 20133608</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 12:59, 19 July 2010
- Description: cell-shape determining protein
| Gene name | mreB |
| Synonyms | divIVB |
| Essential | yes PubMed |
| Product | cell-shape determining protein |
| Function | cell-shape determination |
| MW, pI | 35 kDa, 4.901 |
| Gene length, protein length | 1011 bp, 337 aa |
| Immediate neighbours | mreC, radC |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 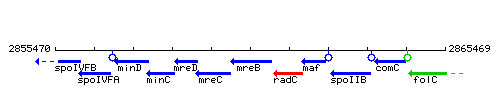
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU28030
Phenotypes of a mutant
essential PubMed, the mutation can be suppressed by inactivation of ponA, ptsI, ccpA PubMed or addition of 5 mM Magnesium to the growth medium PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: ftsA/mreB family (according to Swiss-Prot)
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization:
Database entries
- UniProt: Q01465
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in the labs of Jeff Errington and Boris Görke
- Antibody: available in the Jeff Errington and Peter Graumann labs
Labs working on this gene/protein
Jeff Errington, Newcastle University, UK homepage
Peter Graumann, Freiburg University, Germany homepage
Your additional remarks
References
Reviews
Loclization
Other original publications