Difference between revisions of "ClpX"
(→References) |
|||
| Line 124: | Line 124: | ||
=References= | =References= | ||
| + | ==Reviews== | ||
| + | <pubmed> 19609260 </pubmed> | ||
| + | ==Original Publications== | ||
<pubmed>10809708,9643546,11807061,14679237,18689476,16899079,8973311, 19136590 , 9643546, 18786145 </pubmed> | <pubmed>10809708,9643546,11807061,14679237,18689476,16899079,8973311, 19136590 , 9643546, 18786145 </pubmed> | ||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 14:50, 23 July 2009
- Description: ATP-dependent Clp protease ATP-binding subunit (class III heat-shock protein)
| Gene name | clpX |
| Synonyms | |
| Essential | no |
| Product | ATP-dependent Clp protease ATP-binding subunit |
| Function | protein degradation |
| Metabolic function and regulation of this protein in SubtiPathways: Stress | |
| MW, pI | 46 kDa, 4.645 |
| Gene length, protein length | 1260 bp, 420 aa |
| Immediate neighbours | lonB, tig |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 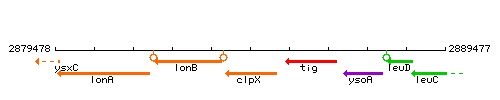
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU28220
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: ATPase/chaperone
- Protein family: clpX chaperone family (according to Swiss-Prot) ClpX (IP004487) InterPro, AAA+ -type ATPase (IPR013093) InterPro (PF07724) PFAM
Extended information on the protein
- Kinetic information:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
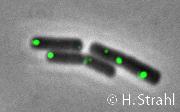
- Localization: cytoplasmic polar clusters, excluded from the nucleoid, induced clustering upon heatshock, colocalization with ClpP Pubmed
Database entries
- Structure: homologue structure resolved 1UM8, structural model of B. subtilis ClpX available from hstrahl
- UniProt: P50866
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant: clpX::kan, clpX::spec and clpX::cat available from the Hamoen] Lab
- Expression vector:
- lacZ fusion:
- GFP fusion: C-terminal GFP fusions (both single copy and 2th copy in amyE locus, also as CFP and YFP variants) available from the Hamoen] Lab
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Leendert Hamoen, Newcastle University, UK homepage
Your additional remarks
References
Reviews
Original Publications
Daniel P Haeusser, Amy H Lee, Richard B Weart, Petra Anne Levin
ClpX inhibits FtsZ assembly in a manner that does not require its ATP hydrolysis-dependent chaperone activity.
J Bacteriol: 2009, 191(6);1986-91
[PubMed:19136590]
[WorldCat.org]
[DOI]
(I p)
Janine Kirstein, Henrik Strahl, Noël Molière, Leendert W Hamoen, Kürşad Turgay
Localization of general and regulatory proteolysis in Bacillus subtilis cells.
Mol Microbiol: 2008, 70(3);682-94
[PubMed:18786145]
[WorldCat.org]
[DOI]
(I p)
James Kain, Gina G He, Richard Losick
Polar localization and compartmentalization of ClpP proteases during growth and sporulation in Bacillus subtilis.
J Bacteriol: 2008, 190(20);6749-57
[PubMed:18689476]
[WorldCat.org]
[DOI]
(I p)
Stephan Zellmeier, Wolfgang Schumann, Thomas Wiegert
Involvement of Clp protease activity in modulating the Bacillus subtilissigmaw stress response.
Mol Microbiol: 2006, 61(6);1569-82
[PubMed:16899079]
[WorldCat.org]
[DOI]
(P p)
Ulf Gerth, Janine Kirstein, Jörg Mostertz, Torsten Waldminghaus, Marcus Miethke, Holger Kock, Michael Hecker
Fine-tuning in regulation of Clp protein content in Bacillus subtilis.
J Bacteriol: 2004, 186(1);179-91
[PubMed:14679237]
[WorldCat.org]
[DOI]
(P p)
Tiina Pummi, Soile Leskelä, Eva Wahlström, Ulf Gerth, Harold Tjalsma, Michael Hecker, Matti Sarvas, Vesa P Kontinen
ClpXP protease regulates the signal peptide cleavage of secretory preproteins in Bacillus subtilis with a mechanism distinct from that of the Ecs ABC transporter.
J Bacteriol: 2002, 184(4);1010-8
[PubMed:11807061]
[WorldCat.org]
[DOI]
(P p)
E Krüger, E Witt, S Ohlmeier, R Hanschke, M Hecker
The clp proteases of Bacillus subtilis are directly involved in degradation of misfolded proteins.
J Bacteriol: 2000, 182(11);3259-65
[PubMed:10809708]
[WorldCat.org]
[DOI]
(P p)
U Gerth, E Krüger, I Derré, T Msadek, M Hecker
Stress induction of the Bacillus subtilis clpP gene encoding a homologue of the proteolytic component of the Clp protease and the involvement of ClpP and ClpX in stress tolerance.
Mol Microbiol: 1998, 28(4);787-802
[PubMed:9643546]
[WorldCat.org]
[DOI]
(P p)
U Gerth, A Wipat, C R Harwood, N Carter, P T Emmerson, M Hecker
Sequence and transcriptional analysis of clpX, a class-III heat-shock gene of Bacillus subtilis.
Gene: 1996, 181(1-2);77-83
[PubMed:8973311]
[WorldCat.org]
[DOI]
(P p)