Difference between revisions of "FadH"
| Line 80: | Line 80: | ||
* '''Swiss prot entry:''' [http://www.uniprot.org/uniprot/O34717 O34717] | * '''Swiss prot entry:''' [http://www.uniprot.org/uniprot/O34717 O34717] | ||
| − | * '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu | + | * '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu:BSU14060] |
* '''E.C. number:''' [http://www.expasy.org/enzyme/1.3.1.34 1.3.1.34] | * '''E.C. number:''' [http://www.expasy.org/enzyme/1.3.1.34 1.3.1.34] | ||
Revision as of 07:29, 25 June 2009
- Description: 2,4-dienoyl-CoA reductase
| Gene name | fadH |
| Synonyms | ykuF |
| Essential | no |
| Product | 2,4-dienoyl-CoA reductase |
| Function | fatty acid degradation |
| MW, pI | 26 kDa, 6.495 |
| Gene length, protein length | 762 bp, 254 aa |
| Immediate neighbours | ykuE, fadG |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 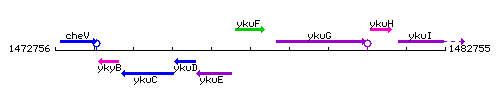
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU14060
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Trans-2,3-didehydroacyl-CoA + NADP+ = trans,trans-2,3,4,5-tetradehydroacyl-CoA + NADPH (according to Swiss-Prot)
- Protein family: 2,4-dienoyl-CoA reductase subfamily (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization:
Database entries
- Structure:
- Swiss prot entry: O34717
- KEGG entry: [3]
- E.C. number: 1.3.1.34
Additional information
Expression and regulation
- Operon:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Hiroshi Matsuoka, Kazutake Hirooka, Yasutaro Fujita
Organization and function of the YsiA regulon of Bacillus subtilis involved in fatty acid degradation.
J Biol Chem: 2007, 282(8);5180-94
[PubMed:17189250]
[WorldCat.org]
[DOI]
(P p)